Clinical Management of Advanced Prostate Cancer: Where Does Radiopharmaceutical Therapy Fit in the Treatment Algorithm? | Journal of Nuclear Medicine
Clinical Management of Advanced Prostate Cancer: Where Does Radiopharmaceutical Therapy Fit in the Treatment Algorithm? | Journal of Nuclear Medicine
Research ArticleContinuing Education
Journal of Nuclear Medicine May 2024, 65 (5) 679-685; DOI: https://doi.org/10.2967/jnumed.123.267006
Abstract
Most men with newly appreciated metastatic prostate cancer are optimally treated with a backbone consisting of androgen receptor–directed therapy with or without taxane chemotherapy. Despite improvements in disease outcomes, prostate cancer remains an extremely heterogeneous disease with variable mechanisms of therapeutic resistance. As a result, it remains a leading cause of cancer-related death in men. Radiopharmaceutical therapy has emerged as an alternative, non–androgen receptor–directed treatment modality for metastatic castration-resistant prostate cancer that impacts patient survival and represents a potentially more personalized approach. In this review, we aim to outline the current treatment landscape for metastatic prostate cancer with a focus on radiopharmaceutical therapy, specifically 177Lu-PSMA-617. In addition, we illustrate various clinical challenges with 177Lu-PSMA-617 treatment to date and explore investigative efforts to leverage radiopharmaceutical therapies as part of combination regimens or earlier in the treatment algorithm to further improve patient outcomes. Finally, we introduce ongoing studies of alternative radiopharmaceutical therapies in metastatic prostate cancer that may be incorporated into the treatment algorithm pending further study.
Most men with newly appreciated metastatic prostate cancer are optimally treated with a backbone consisting of androgen receptor (AR)–directed therapy with or without taxane chemotherapy. Despite improvements in disease outcomes, prostate cancer remains an extremely heterogeneous disease with variable mechanisms of therapeutic resistance. As a result, it remains a leading cause of cancer-related death in men. Radiopharmaceutical therapy has emerged as an alternative, non–AR-directed treatment modality for metastatic castration-resistant prostate cancer (mCRPC) that impacts patient survival and represents a potentially more personalized approach. In this review, we aim to outline the current treatment landscape for metastatic prostate cancer with a focus on radiopharmaceutical therapy, specifically 177Lu-PSMA-617. In addition, we illustrate various clinical challenges with 177Lu-PSMA-617 treatment to date and explore investigative efforts to leverage radiopharmaceutical therapies as part of combination regimens or earlier in the treatment algorithm to further improve patient outcomes. Finally, we introduce ongoing studies of alternative radiopharmaceutical therapies in metastatic prostate cancer that may be incorporated into the treatment algorithm pending further study.
METASTATIC HORMONE-SENSITIVE PROSTATE CANCER
Introduction
Initial treatment of prostate cancer with androgen deprivation therapy (ADT), either surgically with orchiectomy or medically with a luteinizing hormone–releasing hormone agonist (leuprolide, goserelin, triptorelin, and histrelin) or antagonist (degarelix and relugolix), leads to suppression of testosterone production from the testes and an initial disease response. Since 2015, ADT intensification has demonstrated significant survival benefits in patients in several landmark phase III clinical trials, leading to a change in the standard of care.
Intensification of ADT for Metastatic Hormone-Sensitive Prostate Cancer (mHSPC)
Three classes of agents have been demonstrated to improve overall survival in patients with mHSPC when added to either medical or surgical castration: chemotherapy, AR inhibitors, and androgen synthesis inhibitors. Docetaxel is an intravenous taxane chemotherapy that stabilizes microtubules and results in cell cycle arrest and apoptosis (1). Enzalutamide, apalutamide, and darolutamide are oral nonsteroidal antiandrogens that inhibit AR signaling through competitive inhibition of androgen binding to the AR, inhibition of AR translocation to the cellular nucleus, and inhibition of the transcriptional activation of AR once in the nucleus (2). Abiraterone acetate is an oral inhibitor of CYP17A1. The enzymatic function of CYP17A1 as 17-α-hydroxylase and 17,20-lyase is responsible for the conversion of steroid precursors into androgen precursors in the testes, adrenal glands, peripheral adipose tissue, and prostate cancer cells. This action within the adrenal gland can result in overproduction of mineralocorticoids, causing symptoms of hypertension, hyponatremia, and hypokalemia. Coadministration of prednisone is required to prevent mineralocorticoid excess.
Each of these drugs has been tested in combination with ADT versus ADT alone in the treatment of mHSPC in multiple studies with similar designs (Fig. 1). These studies demonstrated that intensification of therapy for mHSPC results in significant improvements in overall survival (Table 1) (3–9). The CHAARTED study included a preplanned subgroup analysis that showed that only patients with high-volume disease (defined as the presence of visceral metastases or ≥4 bone metastases with ≥1 outside the vertebral bodies and pelvis) benefitted from docetaxel intensification (10). Although the STAMPEDE study did not assess for disease volume, post hoc analysis suggested that patients benefitted from docetaxel regardless of disease volume (11). The LATITUDE trial enrolled patients with de novo mHSPC and also demonstrated a survival benefit despite the higher-risk patient population (5). More recently, triplet therapy has also emerged as an option for patients with de novo mHSPC. The ARASENS trial examined ADT/docetaxel versus ADT/docetaxel/enzalutamide, with a hazard ratio of 0.68 (P < 0.001) favoring the triplet (12). The PEACE-1 trial similarly tested ADT/docetaxel versus ADT/docetaxel/abiraterone and showed a hazard ratio of 0.82 (P = 0.030) favoring the triplet (13). In addition to survival, quality-of-life metrics were analyzed in many of these studies. With the exception of docetaxel, patient-reported quality-of-life metrics were maintained or improved with intensification of therapy (14–17). Although docetaxel does negatively impact the quality of life during administration, the quality of life improves after conclusion of chemotherapy and eventually surpasses that of ADT monotherapy (14).
TABLE 1.
Summary of Phase III Clinical Trials for Intensification of Upfront Therapy for Treatment of mHSPC
On the basis of this evidence, frontline treatment for mHSPC should involve intensification with either doublet or triplet therapy. Real-world use of ADT intensification has been slowly increasing, though most men with mHSPC continue to be treated with ADT monotherapy (18). Most patients treated with intensification will receive doublet therapy, with triplet therapy reserved for patients with de novo metastatic disease who are symptomatic and have preserved Eastern Cooperative Oncology Group performance status.
METASTATIC MCRPC
Introduction
Resistance to ADT-based therapies inevitably develops, resulting in mCRPC, defined as disease progression documented by a composite of findings including rising prostate-specific antigen (PSA) or radiographic disease progression on imaging—historically, CT, MRI, and 99mTc bone scintigraphy, and, more recently, prostate-specific membrane antigen (PSMA) PET/CT—despite testosterone levels below 50 ng/dL, or 1.7 nmol/dL (19). Most mechanisms of resistance in mCRPC continue to leverage AR signaling such as AR gene amplification and overexpression, ligand-binding domain mutations, structural rearrangements, variant AR receptors that are constitutively active, and alterations in androgen biosynthesis pathways (20). Although mCRPC is historically a refractory disease state with limited survival, over the past 20 y several therapeutic agents have received U.S. Food and Drug Administration (FDA) approval, each with the potential to modestly improve overall survival (21).
The approval of various therapies for mCRPC has created a dilemma for clinicians given the paucity of evidence regarding the optimal treatment sequence. The prospective clinical trials that led to the various drug approvals often compared the investigational agent with a control group that was not consistent with the contemporary standard of care. In addition, many of these trials leveraged surrogate endpoints such as radiographic progression-free survival, which, though it provides insight into the efficacy of the compared interventions, does not inform about superiority in terms of sequence. With a lack of prospective studies directly informing treatment sequence, we are left with retrospective analyses whose conclusions are at best hypothesis-generating because of a lack of prospective randomization to address accidental bias. Oncologists are left with other variables to inform sequencing decisions, including disease characteristics (i.e., symptomatic vs. asymptomatic, volume and sites of disease, pace of growth, molecular features), patient characteristics (i.e., prior therapies received, functional status, goals of care, comorbid conditions, concomitant medications), drug characteristics (i.e., side effect profile, associated biomarker, cost, availability), and physician characteristics (i.e., individual experience, individual interpretation of available data, practice setting). This lack of prospective sequence studies leaves clinicians without a universally accepted treatment algorithm for the management of patients with mCRPC.
Taxane Chemotherapy and AR-Signaling Inhibitors (ARSIs)
Both docetaxel and cabazitaxel are taxane chemotherapeutics that are FDA-approved on the basis of superior survival compared with mitoxantrone, with cabazitaxel demonstrating activity in patients progressing on docetaxel (22,23).
Although sequential use of an ARSI and taxane chemotherapy had once represented most of the mCRPC treatment landscape, increasing numbers of patients develop mCRPC after treatment intensification with an ARSI. Use of an alternative ARSI on progression has been associated with suboptimal outcomes, suggesting cross resistance in most patients (24,25). In fact, the prospective CARD trial observed a significant improvement in survival with cabazitaxel compared with either enzalutamide or abiraterone in men with mCRPC who were previously treated with docetaxel and either abiraterone or enzalutamide (26).
Bone-Targeted Therapy
Radiotherapeutics in mCRPC initially took the form of bone-targeted radionuclides. Metastatic prostate cancer demonstrates significant bone tropism, often developing osteoblastic lesions. These lesions can lead to significant morbidity for the patient because of pain and fracture, which compromise the quality of life. Radionuclides that follow biochemical pathways similar to that of calcium have been studied in patients with mCRPC with painful bony metastases. 89Sr, a bone-targeted β-emitter, demonstrated significant palliation of pain but not a significant difference in survival compared with external-beam irradiation (27). Compared with docetaxel alone, combination 89Sr and docetaxel demonstrated only a modest improvement in bony clinical disease progression and did not demonstrate superior survival or improvement in skeleton-related events in mCRPC (28). 223Ra, also a calcium mimetic, is an α-emitter, allowing for more localized and higher-energy radiation resulting in a therapeutically effective radioactive dose that spares healthy bone marrow compared with β-emitters (29). The phase III ALSYMPCA study in men with bone mCRPC demonstrated a significant improvement in median overall survival (mOS) of 14.9 versus 11.3 mo along with a significantly prolonged time to the first symptomatic skeletal event and decreased adverse events with 223Ra compared with placebo (30). As a result, the FDA approved 223Ra for the treatment of men with mCRPC, symptomatic bone metastases, and no known visceral metastatic disease (31). 223Ra is currently limited to use as monotherapy because combination with the ARSI abiraterone was associated with no further survival advantage and an increased risk of fractures (32).
Vaccine Therapy
Sipuleucel-T is an autologous vaccine consisting of peripheral-blood mononuclear cells activated with a fusion protein consisting of prostatic acid phosphatase and granulocyte-macrophage colony-stimulating factor. The phase III IMPACT trial demonstrated an improvement in mOS of 25.8 versus 21.7 mo compared with placebo (33). Of note, it did not demonstrate improvement in time to objective disease progression or PSA response from baseline, which has led to its lack of use broadly. The FDA approval restricted its use in patients with asymptomatic or minimally symptomatic mCRPC and no evidence of visceral metastases (34).
Poly(Adenosine Diphosphate-Ribose) Polymerase (PARP) Inhibitors
More recently, the use of PARP inhibitors has established a role in the mCRPC treatment landscape. Trapping of PARP impairs replication fork progression, which induces a DNA damage response. However, the lack of homologous recombination repair (HRR) due to the presence of a genomic alteration in one of the associated DNA repair genes presents an opportunity to induce synthetic lethality (35). Up to 30% of patients with mCRPC carry somatic or germline alterations in DNA HRR genes, the most common of which is BRCA2 (36,37).
The PARP inhibitor olaparib has been FDA-approved as monotherapy in mCRPC with an HRR gene alteration after progression on either enzalutamide or abiraterone. This is based on the phase III PROfound study that demonstrated a mOS benefit of 19.1 versus 14.7 mo (hazard ratio, 0.69; 95% CI, 0.50–0.97) with olaparib compared with alternative enzalutamide or abiraterone after progression on prior enzalutamide or abiraterone. The survival benefit with olaparib was predominantly seen in those with a BRCA alteration, and it remains unclear how much patients benefitted from the PARP inhibitor with an alternative HRR alteration. The TRITON3 phase III trial also studied a PARP inhibitor, rucaparib, in patients with mCRPC and alterations in BRCA1, BRCA2, or ATM after progression on an ARSI. This trial did include docetaxel as a potential treatment option in the control group, and patients with a BRCA alteration had superior median radiographic progression-free survival (mPFS) of 11.2 versus 8.3 mo when compared with docetaxel (38). Therefore, use of a PARP inhibitor is currently applied after progression on an ARSI in those patients with mCRPC who are found to have a BRCA alteration on either somatic or germline next-generation sequencing.
More recently, combination of a PARP inhibitor and ARSI has demonstrated significant benefit in mPFS compared with ARSI alone for patients with mCRPC after progression on ADT. This has led to FDA approval for use of olaparib plus abiraterone for mCRPC with BRCA 1 or BRCA2 gene alteration and talazoparib plus enzalutamide for mCRPC with an HRR gene alteration (39,40). However, the use of these combinations is limited by the fact that most metastatic prostate cancer patients receive ARSI with ADT in the hormone-sensitive setting.
PSMA RADIOPHARMACEUTICAL THERAPY
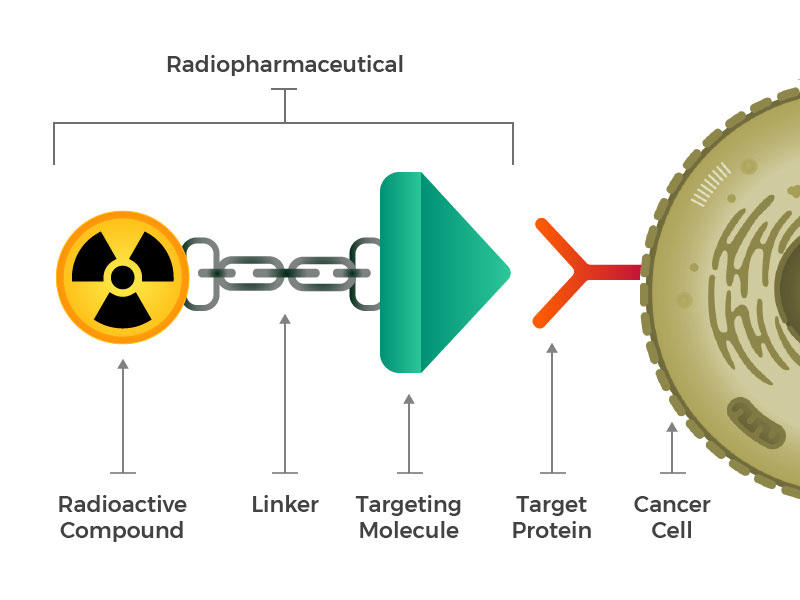
PSMA, also known as glutamate carboxypeptidase II and folate hydrolase 1 and encoded by the FOLH1 gene, is a transmembrane glycoprotein enzyme expressed at the cell surface of more than 90% of prostate cancers at a level up to 100- to 1,000-fold higher than in nonprostate tissues (41). Despite AR regulation of PSMA, expression has also been noted in a subset of AR-negative mCRPC (42). As a result, PSMA-targeted small-molecule and antibody radiopharmaceutical therapy has garnered significant interest.
177Lu-PSMA-617 FDA Approval
177Lu-vipivotide tetraxetan (177Lu-PSMA-617) is a β-emitting small-molecule radioligand with nanomolar affinity for PSMA along with durable accumulation in prostate cancer tumors.177Lu-PSMA-617 in combination with the standard of care was compared with the standard of care alone in men with mCRPC in the international open-label phase III VISION trial. Patients must have experienced disease progression after an ARSI and 1–2 taxane chemotherapies. At a median follow-up of 20.9 mo, the 177Lu-PSMA-617–containing cohort experienced mPFS of 8.7 versus 3.4 mo (hazard ratio, 0.40; 95% CI, 0.29–0.57; P < 0.001) and mOS of 15.3 versus 11.3 mo (hazard ratio, 0.62; 95% CI, 0.52–0.74; P < 0.001) (43). This led to the FDA approval of 177Lu-PSMA-617 for the treatment of men with PSMA-positive mCRPC who have been treated with prior ARSI and taxane-based chemotherapy (44).
Radiopharmaceutical Therapy in mHSPC
Radiopharmaceutical therapy does not have a defined role in the treatment of mHSPC. 177Lu-PSMA-617 was assessed in a retrospective single-center study in patients with mHSPC and demonstrated activity as a single agent, with good tolerability and mPFS of approximately 10 mo (45). A phase I study of patients with oligometastatic mHSPC, defined as 10 or fewer metastases, and PSA doubling times of less than 6 mo explored treatment with 2 cycles of 177Lu-PSMA-617 instead of ADT. The 10 patients treated had an mPFS of 11 mo, with 5 patients experiencing a greater than 50% reduction in PSA level, and there were no grade 3 or greater treatment-related adverse events (46). Radioligand monotherapy will require subsequent study in larger trials to determine whether it may represent an alternative to standard therapy. PSMAddition, a prospective, randomized, phase III study evaluating standard-of-care ADT plus ARSI with or without 6 cycles of 177Lu-PSMA-617 in patients with mHSPC, has completed accrual, and results are eagerly awaited.
Clinical Challenges of 177Lu-PSMA-617 Treatment
Not all patients with metastatic disease will be eligible for 177Lu-PSMA-617. Up to 15%–20% of prostate cancers lose PSMA expression (42). PSMA expression has also been noted to vary across sites of metastases even in patients with PSMA-positive mCRPC (42). To be eligible for 177Lu-PSMA-617 therapy on the VISION trial, patients must have had at least 1 PSMA-positive metastatic lesion defined as showing greater uptake than that of liver parenchyma and no PSMA-negative lesion on 68Ga‐PSMA‐11 or 18F‐DCFPyL PET/CT imaging (43). Worse outcomes have been reported in patients who do not meet the VISION imaging criteria (47). Alternatively, the phase II TheraP trial that compared 177Lu-PSMA-617 with cabazitaxel required a single lesion to have an SUVmax of more than 20 and all measurable lesions to have an SUVmax of more than 10, with no evidence of disease with an SUVmax of less than 10 or mismatch with 18F-FDG PET (48). The timing of PSMA PET evaluation for patient selection remains poorly understood. The Society of Nuclear Medicine and Molecular Imaging consensus statement recommends that PSMA PET be performed within 3 mo of treatment and after any noted disease progression or intervening therapy (49). It is also recommended that either CT or MRI with contrast medium be obtained to identify PSMA-negative disease, specifically in patients with known liver involvement. However, meeting these criteria may be a challenge clinically with regard to insurance approval, especially if a patient undergoes PSMA PET just outside this 3-mo window.
The current role of radiographic monitoring of patients managed with 177Lu-PSMA-617 remains undefined, with no compelling evidence regarding the role of subsequent monitoring with PSMA PET/CT. Though PSMA PET/CT may better visualize PSMA-positive disease, the loss of PSMA expression with radiopharmaceutical therapy may lead to pitfalls with PSMA PET-based imaging. The phase III VISION trial used CT scans and bone scans every 12 wk for disease monitoring during treatment based on Prostate Cancer Clinical Trials Working group 3 recommendations, which outline specific CT and bone scan criteria for radiographic disease assessment (50). However, repeating a PSMA PET/CT scan may be of value if additional doses of 177Lu-PSMA-617 are being considered. Although 177Lu-PSMA SPECT quantitation at 6 wk (dose 2) may predict outcomes to 177Lu-PSMA-617 therapy, it remains an investigational approach and requires validation in larger prospective studies (51).
The VISION study established the standard dosing of 177Lu-PSMA-617 at 7.4 GBq every 6 wk for up to 6 cycles. However, alternative doses and schedules are being investigated. The TheraP trial initiated 177Lu-PSMA-617 at 8.5 GBq and decreased by 0.5 GBq per cycle to 6 GBq for cycle 6 (48). The dosing of radiopharmaceutical therapy is further complicated by the different models of therapeutic development. The radiotherapy model uses dosimetry and prohibits the dose from exceeding known maximum tolerated limits to critical organs, which are theoretic limits extrapolated from external-beam radiotherapy. Alternatively, the drug model uses a phase 1 dose escalation study to determine the maximum tolerated dose, which is more definable but may miss delayed cumulative toxicities (41). The LuPSMA pilot study of men with mCRPC treated with 177Lu-PSMA-617 suggests that the response to treatment can be predicted by the dose to the tumor, as 10 of 11 patients who received less than 10 Gy to the tumor did not have a PSA decline of 50% or more, providing a rationale for patient-specific dosing (52).
PSMA expression is also observed in benign tissues such as the salivary and lacrimal glands; dry mouth was a commonly encountered adverse event with 177Lu-PSMA-617. Other common adverse events included myelosuppression, fatigue, nausea, vomiting, diarrhea, constipation, loss of appetite, and arthralgias. These adverse events may lead to dose interruptions, reductions, or discontinuation. More than 10% of patients on the VISION trial had to discontinue 177Lu-PSMA-617 because of an adverse event (43). Despite the increased incidence of grade 3–4 adverse events, patient-reported outcomes were significantly lower in the 177Lu-PSMA-617–containing group (53). In clinical practice, toxicity assessments can be complicated by the logistics of how the dose is received from the manufacturer. Doses cannot be repurposed for other patients, and treatments must therefore be canceled with advanced notice to the manufacturer, which requires earlier toxicity assessments than what is traditionally conducted for other cancer-directed therapies.
177Lu-PSMA-617 Sequencing in Current Treatment Algorithm
FDA approval of 177Lu-PSMA-617 is currently limited to the treatment of men with PSMA-positive mCRPC who have been treated with an ARSI and taxane-based chemotherapy. Though length of exposure to chemotherapy to qualify for 177Lu-PSMA-617 is not specifically defined, it is typically intended that patients receive treatment until completion, progression, or dose-limiting toxicity (49). The optimal sequencing of 177Lu-PSMA-617 after ARSI and docetaxel remains undefined. As noted earlier, the prospective CARD trial previously observed a significant improvement in mPFS and mOS with cabazitaxel compared with the alternative ARSI in men with mCRPC previously treated with docetaxel and an ARSI (26). As a result, cabazitaxel has traditionally been used once a patient has progressed on prior ARSI and docetaxel. The protocol-permitted standard of care on the VISION trial excludes chemotherapy, immunotherapy, 223Ra, and investigational drugs and therefore does not address sequencing of 177Lu-PSMA-617 and cabazitaxel. The randomized open-label phase II TheraP trial compared 177Lu-PSMA-617 with cabazitaxel, 20 mg/m2 (48). Though the study met its primary endpoint of a difference in PSA reduction of at least 50% from baseline (66% vs. 37%; P < 0.0001), the clinical relevance of this endpoint compared with survival outcomes is questionable. Therefore, the sequencing of 177Lu-PSMA-617 and cabazitaxel after docetaxel remains undefined. On the basis of the improved time to pain progression and patient-reported outcomes, as well as fewer grade 3–4 adverse events than for cabazitaxel in the TheraP trial, it is reasonable to initiate 177Lu-PSMA-617 after progression on an ARSI and docetaxel and before cabazitaxel. This also avoids the potential for complications with myelosuppression that may follow significant taxane chemotherapy exposure, which could limit safe and effective 177Lu-PSMA-617 dosing.
The sequencing with 223Ra is also an important clinical question with a lack of prospective evidence. As stated earlier, although 223Ra was associated with a survival benefit in mCRPC, this study was conducted before the approval of second-generation ARSIs (30). Concerns about dose-limiting myelosuppression similar to those that can occur with taxane chemotherapy exposure may also occur with prior 223Ra use. However, the RALU study retrospectively observed the feasibility of 177Lu-PSMA-617 in patients treated with prior 223Ra without evidence of significant myelosuppression (54).
Another open question is the use of 177Lu-PSMA-617 before docetaxel, in the mCRPC setting. Data from the phase III PSMAfore study (NCT04689828) were recently presented. Eligible patients were taxane-naïve mCRPC patients with progression on prior ARSI and were treated with either 177Lu-PSMA-617 or the alternative ARSI of abiraterone or enzalutamide. The primary endpoint of mPFS was met (12.02 vs. 5.59 mo; hazard ratio, 0.43; 95% CI, 0.33–0.54), though mOS remains similar to date (19.25 vs. 19.55 mo) with crossover permitted. As the control arm was alternative ARSI as opposed to docetaxel chemotherapy, it remains unclear whether 177Lu-PSMA-617 leads to better outcomes when initiated before chemotherapy in taxane-naïve patients (55). A phase II study demonstrated noninferiority of 177Lu-PSMA-617 (dosed at 6.0–7.4 GBq/cycle up to 4 cycles 8–12 wk apart) compared with docetaxel in chemotherapy-naïve mCRPC in terms of PSA response rate (56). In addition, 33.4 mo of follow-up showed a similar mOS, at 19.0 mo (95% CI, 9.5–20.5 mo) versus 15.0 mo (95% CI, 8.1–21.9 mo). This question will be potentially further informed by the ongoing CCTG PR21 phase II study of 177Lu-PSMA-617 (7.4 GBq every 6 wk for up to 6 cycles) versus docetaxel in patients with mCRPC (57). This study will allow crossover and assess the second mPFS and the time to third-line systemic therapy, which should provide better clarity on the proper sequencing of 177Lu-PSMA-617 and taxane chemotherapy.
177Lu-PSMA-617 Combination Regimens
Although currently approved as monotherapy, there is concern that the efficacy of 177Lu-PSMA-617 is currently limited by heterogeneity of tumor PSMA expression, specifically in micrometastatic disease not seen on imaging. Several studies are under way to see whether 177Lu-PSMA-617–containing combination therapy further improves efficacy and outcomes in patients with mCRPC. The ENZA-p phase II study (NCT04419402) is investigating the combination of 177Lu-PSMA-617 and enzalutamide in taxane-naïve mCRPC (58). With increasing use of ARSIs in the castration-sensitive setting, the utility of this combination in the mCRPC will be limited in the current treatment landscape, though patients who received prior abiraterone treatment are allowed on study. The phase I/II LuCAB trial (NCT05340374) is assessing the safety and preliminary efficacy of combination 177Lu-PSMA-617 with cabazitaxel after progression on ARSI and docetaxel (59). On the basis of prior evidence of enhanced PARP inhibitor activity when combined with radiotherapy, the phase I LuPARP trial is studying the safety of combination olaparib and 177Lu-PSMA-617 (60). Numerous studies are assessing the safety and efficacy of combination immunotherapy and 177Lu-PSMA-617 in hopes of improving on the limited single-agent efficacy of immune checkpoint inhibitors in mCRPC to date (61,62).
Alternative Lutetium Radiopharmaceutical Therapies
Another selective 177Lu-labeled PSMA-targeting small molecule is PSMA ligand for imaging and therapy (PSMA-I&T). 177Lu-PSMA-I&T was compared with 177Lu-PSMA-617 in a study of safety, kinetics, and dosimetry. Both demonstrated favorable safety profiles in patients with mCRPC. Despite a lower median effective half-life and median residence time for 177Lu-PSMA-I&T, the mean absorbed dose was similar and there was large interpatient variability in terms of dosimetry parameters (63). 177Lu-PSMA-I&T remains investigational, pending the results of ongoing studies. The phase III SPLASH trial compared 177Lu-PSMA-I&T (177Lu-PNT2002) (6.8 GBq every 8 wk up to 4 cycles) with enzalutamide or abiraterone in patients with mCRPC previously treated with an AR pathway inhibitor as well as prior docetaxel if given in the castration-sensitive setting (NCT04647526). A press release indicated that 177Lu-PNT2002 demonstrated statistically significant improvement in rPFS (64). The phase III ECLIPSE trial is also comparing 177Lu-PSMA-I&T (7.4 GBq every 6 wk up to 4 cycles) with enzalutamide or abiraterone in a similar patient population (NCT05204927).
Nonlutetium Radiopharmaceutical Therapies
Over a quarter of patients saw no decrease in PSA with 177Lu-PSMA-617 on the VISION trial, and 10% had progressive disease as the best overall response (43). Therefore, alternative radionuclides to 177Lu are under investigation. α-emitters deliver significantly higher energy than β-emitters but in a more limited range, which minimizes radiation exposure to adjacent normal tissue (65). Examples of α-emitters under study include 255Ac-PSMA-617 and 255Ac-PSMA-I&T.
CONCLUSION
Metastatic prostate cancer remains incurable despite recent therapeutic advances, highlighting the ongoing unmet need for new therapeutic options. Radiopharmaceutical therapy represents a novel modality with established efficacy that carries current and future therapeutic implications in metastatic prostate cancer. However, several clinical challenges persist in terms of its use. Though its approval is currently limited to 177Lu-PSMA-617 treatment of mCRPC that has progressed on ARSI and taxane chemotherapy, the treatment algorithm will likely continue to evolve. Numerous studies are under way that explore 177Lu-PSMA-617 at alternative dosages, use in earlier stages of prostate cancer, and use in combination with other approved therapies. Alternative radioisotopes such as α-emitters may further expand the prostate cancer therapeutic landscape and improve patient outcomes.
Footnotes
Learning Objectives: On successful completion of this activity, participants should have (1) improved their understanding and management of advanced prostate cancer; (2) gained a better understanding of the challenges in sequencing therapies in metastatic castration-resistant prostate cancer; and (3) gained a better appreciation for the evolving role of 177Lu-PSMA-617 in advanced prostate cancer.
Financial Disclosure: Dr. Viscuse has received a research grant from Bicycle Therapeutics. Dr. Devitt has received research grants from Seagen, Propella Therapeutics, Arvinas, Merck, and Kangpu. Dr. Dreicer is a consultant for Merck, Hinova, Pfizer, Sanofi Genzyme, Bayer, and Janssen. The authors of this article have indicated no other relevant relationships that could be perceived as a real or apparent conflict of interest.
CME Credit: SNMMI is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to sponsor continuing education for physicians. SNMMI designates each JNM continuing education article for a maximum of 2.0 AMA PRA Category 1 Credits. Physicians should claim only credit commensurate with the extent of their participation in the activity. For CE credit, SAM, and other credit types, participants can access this activity through the SNMMI website (http://www.snmmilearningcenter.org) through May 2027.
Published online Apr. 11, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
- Received for publication January 9, 2024.
- Revision received March 25, 2024.
Comments
Post a Comment