Real-World Experience with 177Lu-PSMA-617 Radioligand Therapy After Food and Drug Administration Approval | Journal of Nuclear Medicine
Real-World Experience with 177Lu-PSMA-617 Radioligand Therapy After Food and Drug Administration Approval | Journal of Nuclear Medicine
Journal of Nuclear Medicine May 2024, 65 (5) 735-739; DOI: https://doi.org/10.2967/jnumed.123.266842
Abstract
We report our initial real-world experience with 177Lu-PSMA-617 radioligand therapy.
Methods: We performed a retrospective review of patients treated with 177Lu-PSMA-617. Pretreatment PSMA PET, laboratory findings, overall survival, a fall in prostate-specific antigen by 50% (PSA50), and toxicities were evaluated.
Results: Ninety-nine patients were included. Sixty patients achieved a PSA50. Seven of 18 (39%) patients who did not meet the TheraP PSMA imaging criteria achieved a PSA50. Nineteen of 31 (61%) patients who did not meet the VISION laboratory criteria achieved a PSA50. Sixty-three patients had a delay or stoppage in therapy, which was due to a good response in 19 patients and progressive disease in 14 patients. Of 10 patients with a good response who restarted treatment, 9 subsequently achieved a PSA50 on retreatment. The most common toxicities were anemia (33%) and thrombocytopenia (21%).
Conclusion: At our center, patients who did not meet the TheraP PSMA imaging criteria or the VISION laboratory criteria benefited from 177Lu-PSMA-617 radioligand therapy.
Prostate cancer is the most common cancer and second leading cause of cancer-related death in men in the United States (1). Metastatic castration-resistant prostate cancer remains fatal, despite the availability of several classes of therapy that delay disease progression and prolong life (2,3). Prostate-specific membrane antigen (PSMA) is a transmembrane protein with high expression in prostatic epithelium and can be targeted for both imaging and treatment (4,5).
In 2022, the Food and Drug Administration approved 177Lu-PSMA-617 (177Lu-vipivotide tetraxetan [Pluvicto; Novartis]) based on the VISION trial for the treatment of patients with PSMA-positive metastatic castration-resistant prostate cancer who have been treated with androgen receptor pathway inhibitors and taxane-based chemotherapy (6). The VISION trial demonstrated that 177Lu-PSMA-617 prolongs progression-free survival (PFS) and overall survival (OS) when added to standard care. The TheraP trial is a phase 2 study evaluating patients progressing on androgen receptor pathway inhibitor and docetaxel (7). Patients were randomized to receive either 177Lu-PSMA-617 or cabazitaxel, and the study demonstrated that 177Lu-PSMA-617 resulted in greater PSA50 (a fall in prostate-specific antigen [PSA] by 50%) response rates and improved radiographic PFS.
We report our initial experience treating patients with 177Lu-PSMA-617 at our institution after Food and Drug Administration approval. These results demonstrate the real-world outcomes of metastatic castration-resistant prostate cancer patients, and we aim to compare our results with those of the VISION trial.
MATERIALS AND METHODS
We performed a retrospective review of patients who received 177Lu-PSMA-617 therapy at our institution as standard clinical care; this retrospective study was approved by the local institutional review board, and the need for informed consent was waived. Patients were treated with up to 6 cycles of 177Lu-PSMA-617 obtained commercially. All patients received 7.4 GBq (200 mCi) ± 10% per cycle.
Imaging Eligibility
All patients underwent pretreatment PSMA PET within 6 mo of treatment. Patients were deemed eligible for PSMA radioligand therapy (RLT) based on the VISION criteria (8). We retrospectively determined whether each patient would have qualified for the TheraP trial on the basis of PSMA PET alone (Supplemental Table 2; supplemental materials are available at http://jnm.snmjournals.org). Because 18F-FDG PET was not available as a screening tool, the full TheraP criteria were not applied. VISION and TheraP PSMA imaging criteria are demonstrated in Supplemental Table 1.
Imaging and Laboratory Evaluation
Patients were imaged between cycles 2 and 4 and roughly 4 wk after cycle 6 using cross-sectional imaging (CT or MRI) during treatment and every 12 wk after treatment. PSA levels were checked the day of each treatment and at 3-wk intervals between therapies. Complete blood counts, creatinine, and liver enzymes were checked every 6 wk throughout therapy. Patients were seen by nuclear medicine physicians and advanced associate practitioners to evaluate for toxicity.
Statistical Analysis
Demographic and clinical characteristics, laboratory values, and posttreatment outcomes were reviewed and compared. RECIST 1.1 and the Common Terminology Criteria for Adverse Events, version 5.0, were used to evaluate radiographic response and to grade treatment toxicities, respectively. For outcome assessments, OS, objective response rate by RECIST 1.1, PSA PFS, and PSA response were assessed. We calculated the percentage of patients with PSA50 among those who did and did not meet the PSMA imaging and laboratory criteria from VISION and TheraP. PSA PFS was censored by follow-up time and death, and OS was censored by follow-up time. We evaluated the percentage of patients who would have met the criteria for enrollment in the VISION trial on the basis of imaging criteria, required prior treatments, and laboratory evaluation. The Kaplan–Meier method was used to estimate event time distributions, and log-rank tests were used for group comparisons. For comparison between groups, a restricted mean survival was calculated, and for analysis, OS was truncated at 19.4 mo and PFS was truncated at 11.6 mo. A P value of less than 0.05 was considered statistically significant. All statistical analyses were done using R, version 4.3.1.
RESULTS
In total, 99 patients were treated between June 2022 and June 2023 (Table 1). The patients received a median of 3 (range, 1–6) cycles of 177Lu-PSMA-617. The median follow-up time was 7.5 mo. Sixty (60%; 95% CI, 0.50–0.69) patients achieved a PSA50. Sixty-seven (67%; 95% CI, 0.57–0.75) patients achieved a fall in PSA by 30%. The average best PSA response was 48% ± 54% (Fig. 1). Broken down by site of metastasis, the average PSA response in patients with bone metastasis was 47% (n = 95); with nodal metastasis, 47% (n = 71); with bone and nodal disease, 48% (n = 67); and with visceral metastasis, 47% (n = 37).
TABLE 1.
Patient Characteristics
Thirty-six (36%) patients received 177Lu-PSMA-617 on the standard 6-wk schedule. Sixty-three patients had a delay or stoppage in therapy (Supplemental Fig. 1). The most common reasons for delay or stoppage were a good response (19 patients) and progressive disease (14 patients). Additionally, in 12 patients, treatment was delayed because of bone marrow toxicity; in 9 patients, because of patient decline; in 3 patients, because of dry mouth; in 2 patients, because of drug supply problems; and in 4 patients, because of other issues (spine surgery, seizures, myocardial infarction, and rib fractures). In 19 patients in whom the treatment was stopped early because of a good response, the median number of cycles that these patients received before the determination of response was 3 (range, 1–5). RLT was restarted in 19 patients who had treatment delay. Broken down by reason for stopping, the median interval between stopping and restarting RLT (only for patients who had restarted) was 3.9 mo (range, 2.4–5.2 mo) in patients with a good response (10 patients), 4.6 mo (range, 3.7–6.1 mo) in patients with bone marrow toxicity (3 patients), and 3.0 mo (range, 2.8–5.3 mo) in patients with other issues (6 patients). In 19 patients whose RLT was stopped early because of a good response, 17 achieved a PSA50 and 11 achieved a fall in PSA by 90%. In the 2 patients who did not achieve a PSA50, one was not a PSA secretor and the other had a marked response on posttreatment imaging, with a 39% decrease in PSA. Ten patients were restarted on PSMA RLT, and of those, 9 achieved a PSA50 and 4 achieved a fall in PSA by 90%. Treatment was not restarted in the remaining 9 patients, because of continued disease control.
Toxicity Evaluation
The average white blood count decreased by 35% ± 40%, and 7 (7%) patients developed grade 3 or 4 leukopenia (Supplemental Table 3). The average platelet count decreased by 42% ± 34%, and 21 (21%) patients developed grade 3 or 4 thrombocytopenia. The average hemoglobin decreased by 20% ± 24%, and 33 (33%) patients developed grade 3 anemia. Forty-three patients developed dry mouth on treatment, 8 of which were grade 2.
Seventeen patients had baseline anemia (hemoglobin < 9 g/dL), of whom 16 received transfusions before PSMA RLT. Overall, 44 patients received blood transfusions during treatment. Eleven patients had thrombocytopenia (platelets < 100 × 109/L) at baseline, and 3 patients developed a bleeding complication. Six patients had leukopenia (white blood cell count < 2.5 × 109/L) at baseline, and no patients developed a neutropenic fever during treatment. Four patients had an estimated glomerular filtration rate less than 50 mL/min before treatment, and 1 patient went on dialysis because of progressive obstructive uropathy, which was not considered therapy-related.
Impact of Eligibility
The PSMA PET imaging eligibility criteria based on the VISION trial were met in 99 (100%) patients. Eighty-one (81%) patients fulfilled the TheraP PSMA imaging criteria. Of the 18 patients who did not meet the TheraP PSMA imaging criteria, 7 (39%) achieved a PSA50 and 8 (44%) achieved a fall in PSA by 30%. Sixty-eight (68%) patients met the VISION laboratory criteria. Thirty-one (31%) patients did not meet the laboratory criteria. Of the 31 patients who did not meet the laboratory criteria, 19 (61%) achieved a PSA50 and 20 (64%) achieved a fall in PSA by 30%.
Of the 31 patients who did not meet the VISION laboratory criteria, 4 patients had grade 3 or 4 toxicity at baseline, and 13 additional patients (17 in total, 55%) developed grade 3 or 4 toxicities during treatment. Of the 68 patients who met the VISION laboratory criteria, no patient had grade 3 or 4 toxicity at baseline, and 22 patients (32%) developed grade 3 or 4 toxicity during treatment.
Radiographic Response and PFS
Forty-one patients had RECIST 1.1 measurable disease, of whom 10 had progressive disease, 18 had stable disease, 12 had a partial response, and 1 had a complete response (objective response rate, 31%). The restricted mean PSA PFS was 5.8 mo (95% CI, 5.0–6.5 mo) (Fig. 2). The restricted mean PSA PFS for those who met the TheraP PSMA imaging criteria was 6.0 mo (95% CI, 5.2–6.8 mo), and the restricted mean PSA PFS for those who did not meet the TheraP PSMA imaging criteria was 4.8 mo (95% CI, 3.1–6.5 mo) (P = 0.20) (Fig. 3). The restricted mean PSA PFS for those who met the VISION laboratory criteria was 5.7 mo (95% CI, 4.8–6.7 mo) versus 6.0 mo (95% CI, 4.6–6.6 mo) for those who did not meet the VISION laboratory criteria (P = 0.66) (Fig. 4).
FIGURE 3.
(A) Restricted mean OS in patients who did not fulfill TheraP PSMA imaging criteria was 12.7 mo, vs. 12.4 mo in patients who met TheraP PSMA imaging criteria. (B) Restricted mean PSA PFS in patients who did not fulfill TheraP PSMA imaging criteria was 4.8 mo, vs. 6.0 mo in patients who met TheraP PSMA imaging criteria.
FIGURE 4.
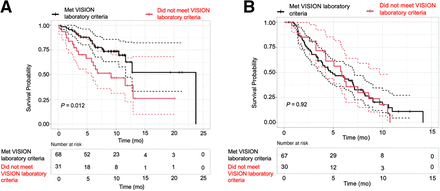
(A) Restricted mean OS in patients who met VISION laboratory criteria was 13.9 mo, vs. 9.7 mo in patients who did not. (B) Restricted mean PSA PFS in patients who met VISION laboratory criteria was 5.7 mo, vs. 6.0 mo in patients who did not. Patients with baseline laboratory issues had similar PSA response but decreased OS, compared with patients who did not have baseline laboratory issues.
OS
The median OS was 12.7 mo. The restricted mean OS was 12.5 mo (95% CI, 10.7–14.2 mo) (Fig. 2). The restricted mean OS for those who met the TheraP PSMA imaging criteria was 12.7 mo (95% CI, 10.8–14.7 mo) versus 12.4 mo (95% CI, 8.8–16.1 mo) for those who did not meet the TheraP PSMA imaging criteria (P = 0.88) (Fig. 3). The restricted mean OS for those who met the VISION laboratory criteria was 13.9 mo (95% CI, 11.7–16.0 mo) versus 9.7 mo (95% CI, 6.9–12.6 mo) for those who did not meet the VISION laboratory criteria (P = 0.022) (Fig. 4).
DISCUSSION
We report our initial experience with 177Lu-PSMA-617 RLT. In a cohort of 99 metastatic castration-resistant prostate cancer patients previously treated with an androgen receptor pathway inhibitor and taxane chemotherapy, PSMA RLT achieved a PSA50 response rate of 60% and an objective response rate of 31%; the most common side effects were anemia and leukopenia. Patients who did not meet the VISION laboratory criteria had a similar PSA50 response rate, but had a decreased OS, compared with those who did, and patients who did not meet the TheraP PSMA imaging criteria had a similar PSA50 response rate and OS to those who did.
Overall, our PSA response rates mirror the experiences described in the VISION and TheraP trials, with a 60% PSA50 response rate, versus 46% in VISION and 66% in TheraP (6,7), although the median OS was 12.7 mo in our cohort, compared with 15.3 mo in VISION and 19.1 mo in TheraP. Additionally, our hematologic toxicity rates were higher than in VISION or TheraP, which was partially due to our treatment of patients with baseline hematologic dysfunction. Finally, the rate of grade 1/2 dry mouth in our study (54%) is consistent with that in VISION (39%) and TheraP (60%).
One of the main questions with PSMA RLT is what PSMA PET uptake cutoff should be used for patient eligibility (9). The TheraP trial demonstrated that patients with higher uptake have a better PSA response, and there was a higher PSA50 in TheraP than in VISION (7,8,10,11). It is hypothesized that higher 68Ga-PSMA-11 uptake correlates with higher 177Lu-PSMA uptake and therefore better response (12). Although prior work demonstrated a low PSA50 in patients who did not meet the TheraP PSMA imaging criteria (20% PSA50) (13), our findings suggest that these patients may in fact benefit from PSMA RLT, given that 39% of these patients achieved a PSA50. It is not clear why Karimzadeh et al. (13) found a lower PSA50 response rate than we did.
Another issue is that of patients who have baseline marrow or kidney dysfunction. Prospective trials such as VISION and TheraP exclude patients with baseline laboratory abnormalities. In our data, the PSA50 in patients who had laboratory issues was 61% versus 60% in the overall population, although there was a significantly lower OS. Overall, patients with baseline laboratory dysfunction appear to benefit from PSMA RLT but have worse OS. The lower OS is likely related to poorer patient factors rather than decreased efficacy of PSMA RLT, and often it may be clinically beneficial to use PSMA RLT on patients with laboratory dysfunction.
A third issue with PSMA RLT is how to manage patients who have good responses. In the TheraP trial, treatment was stopped in patients in whom a near-complete resolution of activity on posttreatment SPECT was noted. Following these criteria, 7% of patients stopped treatment early (7). Emmett et al. have described holding treatment in patients with greater than a PSA50 response and a partial response on posttreatment imaging, and in their series, the treatment of 35% of patients was stopped early (14). In our cohort, the treatment of 19% of patients was stopped early because of a good response, based on PSA and posttreatment imaging. In our practice, there are no strict criteria for a good response, but a combination of PSA response and response seen on posttreatment imaging was used. Of the 10 patients who subsequently restarted treatment, 9 achieved a PSA50, indicating that the residual tumor remained radiation-sensitive after a treatment delay. This suggests that a treatment delay may be a reasonable approach for patients who have a marked response to treatment, although further work is needed to better understand how best to manage these patients.
Overall, it is important to note that the percentage of patients whose treatment was stopped before a full 6 cycles had been administered is much higher than in the VISION and TheraP trials. At first this might be unexpected, but it is important to note that treatment was not dictated by a trial protocol, as our patients were treated as part of clinical care. The rate of stoppage due to marrow toxicity was likely due to the higher rate of baseline marrow abnormalities in our population. The percentage of patients with treatment stoppage because of a good response was higher than in TheraP but less than reported by Emmett et al. (14).
The main limitations of this study were its single-center and retrospective design, which introduced several biases, and therefore our results can be used only as a basis for hypothesis generation. In addition, our cohort did not undergo 18F-FDG PET, and therefore accurate comparison with the TheraP cohort is not possible. Additionally, the length of follow-up was limited, and the number of patients in subgroups was small, limiting statistical comparisons.
CONCLUSION
In our initial real-world experience with 177Lu-PSMA-617 RLT after Food and Drug Administration approval in the United States, our overall results mirrored those of both the VISION trial and the TheraP trial. Additionally, we showed that patients with lower PSMA PET uptake than in TheraP, and those who may not meet the VISION laboratory criteria, appear to benefit from 177Lu-PSMA-617 RLT. Further work needs to be performed to understand which patients benefit clinically from treatment.
DISCLOSURE
Thomas Hope has grant funding to the institution from Clovis Oncology, GE Healthcare, Lantheus, Janssen, the Prostate Cancer Foundation, Telix, and the National Cancer Institute (R01CA235741 and R01CA212148). He received personal fees from Bayer and BlueEarth Diagnostics and Lantheus and received fees from and has an equity interest in RayzeBio and Curium. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: What is the real-world clinical experience of patients treated with 177Lu-PSMA-617?
PERTINENT FINDINGS: In patients who did not meet the TheraP PSMA PET imaging criteria, 39% achieved a PSA50, and in patients who did not meet the VISION laboratory criteria, 61% achieved a PSA50. Ninety percent of patients who restarted therapy because of a delayed good response achieved a subsequent PSA50.
IMPLICATIONS FOR PATIENT CARE: Patients with uptake that does not meet the TheraP criteria and who have poor baseline laboratory function still appear to benefit from PSMA RLT.
Footnotes
Published online Mar. 14, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
- Received for publication October 19, 2023.
- Revision received February 13, 2024.


Comments
Post a Comment