Established focal therapy—HIFU, IRE, or cryotherapy—where are we now?—a systematic review and meta-analysis | Prostate Cancer and Prostatic Diseases
 |
| Just Get The Bad Parts - Focal therapy ablation patterns; |
Key findings:
- - Strong short-term oncological outcomes: 98% overall survival, 99.3% cancer-specific survival, 98.5% metastasis-free survival
- - Annual biochemical progression rate: 9.4%
- - Post-treatment biopsy outcomes: 22.2% clinically significant cancer detection (8.9% in-field, 12.3% out-of-field)
- - Treatment failure rates: 5% required second FT, 10.5% needed radical therapy, 14.1% composite failure rate
- - Good functional outcomes: Low-moderate impact on sexual function, minimal impact on urinary function
- - No significant differences in outcomes between cryotherapy, HIFU, and IRE
- - Increasing focus on treating clinically significant cancers rather than low-risk disease
- - Shift from hemi-gland to more targeted focal treatments
- - Greater use of MRI and targeted biopsies for patient selection
- - Lack of randomized controlled trials
- - Short median follow-up (27.8 months)
- - Heterogeneous reporting of outcomes
- - Incomplete standardization of patient selection and treatment protocols
The authors conclude that while FT shows promising short-term results, longer follow-up and standardized reporting are needed to better define its role in prostate cancer treatment.
How well does focal therapy work?
- - Focal therapy has shown acceptable rates of positive biopsy (in-field and out-field) and clinically significant cancer, with the rates varying by the focal therapy modality used (HIFU, IRE, or cryotherapy).
- - The pooled rate of requiring a second focal therapy procedure is around 14%, indicating that a substantial proportion of patients may need additional treatment.
- - The pooled rate of transitioning to salvage radical therapy (either radical prostatectomy or radiation therapy) is around 14%, suggesting that focal therapy can be an effective alternative to whole-gland treatment for select patients.
- - The pooled rate of composite treatment failure, defined as the need for salvage whole-gland ablation, salvage radical therapy, salvage hormonal therapy, or transition to watchful waiting, is around 14%.
- - Tumor location, specifically anterior tumors, may impact the outcomes of focal therapy, with some evidence that anterior tumors may be more challenging to manage with a single focal therapy modality.
The available evidence suggests that focal therapy can be a viable option for selected patients with localized prostate cancer, but the outcomes may be influenced by the specific pathological features and tumor characteristics. Further research is needed to optimize patient selection and improve long-term outcomes.
Q&A
1. "What's my specific cancer pattern and how does it affect my suitability for focal therapy?"
Expected response: They should discuss:
- Number and location of positive cores
- Whether cancer is unifocal or multifocal
- MRI findings and their correlation with biopsy results
- Whether you fit typical FT criteria (localized disease, clear target lesion)
2. "What are the surveillance protocols after each treatment option?"
For FT: Regular PSA monitoring, MRI at 6-12 months, and likely mandatory biopsy within first 2 years
For other options: PSA monitoring, with additional tests only if progression suspected
3. "If focal therapy fails, what are my backup options?"
Expected response: About 10.5% need radical treatment later. Other options remain available including:
- Repeat focal therapy (5% need this)
- Radical prostatectomy
- Radiation therapy
4. "How many procedures has your center performed, and what are your specific outcomes?"
Look for:
- Clear reporting of cancer control rates
- Functional outcomes (urinary/sexual function preservation)
- Follow-up protocols
- Experience with salvage treatments if needed
5. "Which focal therapy method would you recommend for my specific case and why?"
They should discuss:
- Tumor location (anterior vs posterior)
- Their experience with different methods
- Center-specific expertise and equipment availability
Study Outline
This study aimed to review the outcomes of established focal therapy (FT) modalities like cryotherapy, high-intensity focused ultrasound (HIFU), and irreversible electroporation (IRE) for treating prostate cancer. Focal therapy only targets the area with cancer, unlike standard treatments that remove the entire prostate.
Research objectives and hypotheses:
The researchers wanted to understand the current state of FT and identify gaps in the evidence that can help design better future studies. They did not have specific hypotheses but wanted to provide an overview of FT outcomes.
Methodology:
The researchers conducted a systematic review and meta-analysis of 49 studies reporting outcomes of FT using cryotherapy, HIFU, and IRE. They extracted data on patient selection, treatment details, and various outcomes like survival, cancer recurrence, and side effects.
Results and findings:
The researchers found that FT had good short-to-medium term outcomes, with high overall survival (98%), cancer-specific survival (99.3%), and metastasis-free survival (98.5%). However, the reporting of outcomes was inconsistent across studies. Around 22.2% of patients had clinically significant cancer detected on biopsies after FT, with 8.9% within the treated area and 12.3% outside. About 14.1% of patients needed further treatment like radical therapy or hormonal therapy.
Discussion and interpretation:
The researchers noted that the field of FT is still evolving, and long-term data is lacking. Standardized reporting of pre-treatment cancer patterns, treatment details, and post-treatment outcomes is needed to better understand the role of FT. Randomized trials comparing FT to standard treatments have been challenging due to patient preferences.
Contributions to the field:
This review provides a comprehensive summary of the current state of established FT modalities, highlighting both the successes and the gaps in the evidence. It can guide future research and trial design in this area.
Achievements and significance:
The review shows that FT can achieve good short-term oncological and functional outcomes, but long-term data and standardized reporting are still needed to firmly establish its role in prostate cancer management.
Limitations and future work:
The main limitations are the heterogeneity across studies and the lack of long-term follow-up data. Future studies should focus on standardized reporting of pre-treatment, treatment, and post-treatment details to better understand the role of FT.
Established focal therapy-HIFU, IRE, or cryotherapy-where are we now?-a systematic review and meta-analysis
Abstract
Introduction
Focal Therapy (FT) is a treatment option for the treatment of limited volume clinically significant prostate cancer (csPCa). We aim to systematically review outcomes of established FT modalities to assess the contemporary baseline and identify gaps in evidence that will aid in further trial and study design.
Methods
We conducted a systematic review and meta-analysis of all primary studies reporting outcomes of FT using cryotherapy, high-intensity focused ultrasound (HIFU), and irreversible electroporation (IRE). We described patient inclusion criteria, selection tools, treatment parameters, and surveillance protocols, and pooled overall survival (OS), cancer-specific survival (CSS), metastasis-free survival (MFS), biochemical progression (BP), biopsy, secondary treatment, sexual, and urinary function outcomes. Composite failure was defined as salvage whole gland ablation, radical treatment, hormonal therapy or transition to watchful waiting.
Synthesis
We identified 49 unique cohorts of men undergoing FT between 2008 and 2024 (21 cryotherapy, 20 HIFU, and 8 IRE). Median follow-up ranged from 6 to 63 months. Pooled OS was 98.0%, CSS 99.3%, and MFS 98.5%. Pooled BP was 9.4%/year. Biopsy was mandated post-FT within 24 months in 36/49 (73.5%) cohorts, with pooled csPCa (GG ≥ 2) rates of 22.2% overall, 8.9% infield, and 12.3% outfield. The pooled rate of secondary FT was 5.0%, radical treatment 10.5%, and composite failure 14.1%. Of 35 studies reporting sexual function, 45.7% reported a low, 48.6% moderate, and 5.7% severe impact. For 34 cohorts reporting urinary function, 97.1% reported a low impact. No differences were noted between cryotherapy, HIFU, or IRE in any of the outcomes.
Conclusion
FT with cryotherapy, HIFU, and IRE is associated with good short-intermediate term oncological and functional outcomes. However, outcome reporting is heterogeneous and often incomplete. Long-term follow-up and standardized reporting are required to better define and report FT outcomes.
Similar content being viewed by others
Introduction
Radical therapy with surgery or radiation is the gold standard for treatment of localized prostate cancer (PCa) [1]. However, it is associated with significant treatment-related sexual, urinary, and bowel morbidity [2]. Focal therapy (FT), where only the area of cancer within the prostate is targeted for destruction, could potentially spare the adjacent critical anatomical structures responsible for sexual, urinary, and bowel function from damage, and thus preserve quality of life. Though it was first proposed in the 2000s, physician adoption of FT was limited prior to the late 2010s.
The majority of prostate cancers are multifocal. However, it is now recognized that not every prostate cancer lesion may need to be treated with the understanding that intermediate- and high-grade (Gleason score ≥3 + 4) prostate cancers are the ones that tend to progress and are clinically significant (csPCa), whereas low-grade cancers have low cancer-specific mortality and metastasis and can be safely monitored on active surveillance [3, 4]. Furthermore, when small, low-grade cancer foci are discounted, the proportion of unifocal csPCa increases substantially [5]. The key to successful FT is thus the accurate identification of such unifocal csPCa lesions within the prostate.
Over the last decade, multiparametric magnetic resonance imaging (mpMRI) has emerged as a key imaging tool of the prostate, being able to detect and locate csPCa lesions [6]. The advent of mpMRI-fusion biopsy has allowed physicians merge mpMRI data with accessible office prostate biopsies [7]. These capabilities have significantly increased the feasibility and interest in FT.
There are now multiple new ablative energy sources and delivery methods being developed for FT. This has resulted in efforts being focused on many early-phase studies rather than definitive trials [8]. Despite more than a decade of investigation, majority of FT reports are those of single-arm cohorts. Furthermore, comparison between FT modalities have also been hampered by different trial designs with varying use of pre- and post-treatment imaging and biopsy since these have been developing practices over the last decade.
Among the multiple FT modalities now available, cryotherapy, high-intensity focused ultrasound (HIFU), and irreversible electroporation (IRE) have the longest history of use. A review of these “established” modalities will help us to identify gaps in evidence that will aid in further trial and study design. In this report, we aim to systematically review oncological and functional outcomes of established focal therapy modalities with a view to assess the contemporary baseline and identify gaps in evidence that will aid in further trial and study design.
Methods
Study selection
This systematic review and meta-analysis was registered on PROSPERO (CRD42024554177) and performed in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Guidelines [9].
An electronic literature search was conducted on PubMed, Embase, and Scopus from inception to May 29, 2024, without language restrictions. Bibliographies of each included study were screened, and a search on Google Scholar using the first and last author of each included study was conducted to ensure inclusion of all relevant studies. Abstracts and full texts were reviewed by two independent investigators, with conflicts resolved by a third investigator.
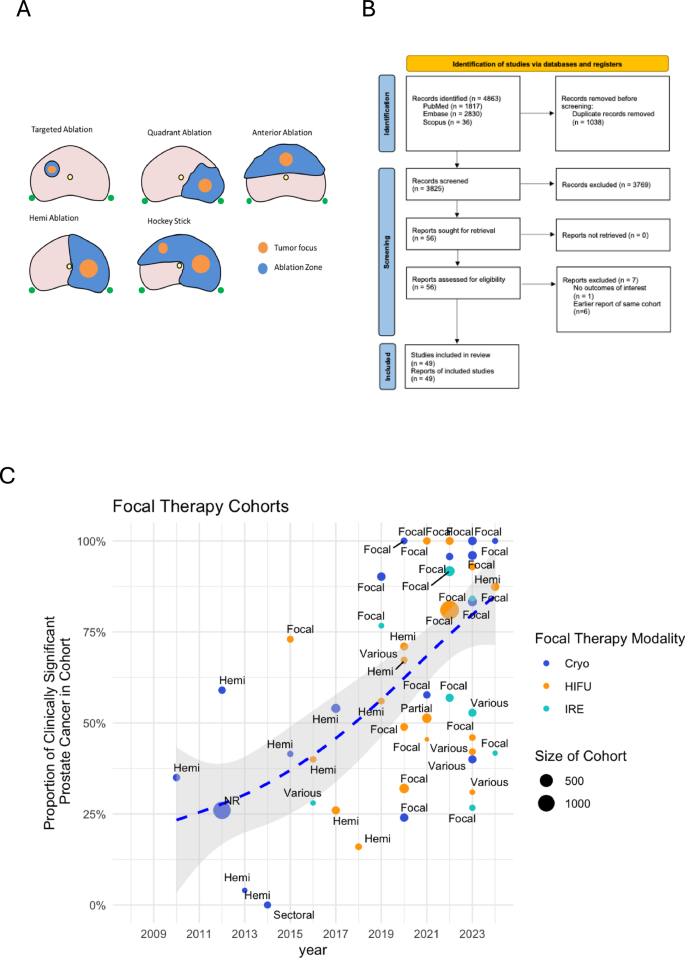
Studies describing either cryotherapy, HIFU, or IRE outcomes with either re-biopsy or progression results were included. Case reports, reviews, conference abstracts, and studies reporting <20 patients were excluded. Duplicate publications were removed. Where a cohort was reported more than once, the latest report was used. We included only cohorts reporting ablation of half the gland or less in this review of focal therapy (Fig. 1A). A standardized data collection template with predefined data fields including study characteristics, patient demographics, and outcomes was used for data extraction by two independent investigators. Risk of bias was assessed using the Newcastle-Ottawa Scale (NOS) detailed in Supplementary Table 1 [10].
A Focal therapy ablation patterns;
B Study Inclusion Flowchart;
C
graph of proportion of clinically significant prostate cancer (csPCa)
as a composition of focal therapy cohorts over time; data points
represent individual cohorts; the dotted blue line is the smoothed
weighted average of proportion of csPCa over time.
Outcome measures
Outcomes extracted included: D’Amico/National Comprehensive Cancer Network (NCCN)/Cancer of the Prostate Risk Assessment (CAPRA) risk groups, Gleason scores and International Society of Urological Pathology (ISUP) grade groups (GG), pre-treatment mpMRI use, pre-treatment targeted/systematic biopsy use, target lesion location, ablation modality, ablation pattern, follow-up MRI use, follow-up biopsy use, follow-up biopsy outcomes, biochemical progression, secondary treatments, overall survival (OS), cancer-specific survival(CSS), metastasis-free survival (MFS), and sexual and urinary functional outcomes.
Due to the varying criteria used to assess functional outcome, we defined the treatment impact of FT on sexual and urinary function arbitrarily by comparison to baseline regardless of the individual measure used. Where function was reduced by <10% compared to baseline, this was classified as low impact. Where function was reduced by 10–30% compared to baseline, this was classified as moderate impact. Where function was reduced by >30% compared to baseline, this was classified as severe impact.
Statistical analysis
Meta-analysis of proportions was carried out for several outcomes. The primary outcome in this study was cancer detection post-FT. This was analyzed in the form of 6 post-FT biopsy outcomes: any cancer, any clinically significant (GG ≥ 2) cancer, in-field cancer, infield clinically significant cancer, outfield cancer, and outfield clinically significant cancer. Secondary outcomes included biochemical progression, treatment failure, acute urinary retention, and urinary tract infection. Outcomes were reported for each of cryotherapy, HIFU, and IRE, and only if >3 studies reporting the outcome in question were available.
For primary outcomes, respective outcome numbers and biopsied patients per study were pooled for each outcome. Random-effects meta-analysis of proportions was conducted and forest plots were generated, with stratification according to time of biopsy if possible. For secondary outcomes, given the short to medium-term follow-up in all studies, the lack of Kaplan-Meier curves in most studies for accounting of time-varying status, as well as low overall event rates, OS, CSS, and MFS were synthesized as proportions for meta-analysis. To account for the significant variation of follow-up time for biochemical progression between studies, and given that this was the significant oncological outcome measure for studies not using mandatory repeat biopsy as part of the protocol given the medium-term follow-up, we assumed that biochemical progression occurred linearly and averaged it over the follow-up time for the purpose of data synthesis, effectively generating an estimate of average biochemical progression rate per year. Forest plots for all outcomes were displayed alongside 95% confidence intervals (95%CI). Heterogeneity was considered low, moderate, or considerable for I2 values < 40%, 40–75%, and >75%, respectively. Funnel plot symmetry was visually assessed for publication bias.
Synthesis
We reviewed 56 original articles on the topic of cryotherapy, HIFU, or IRE FT after excluding systematic reviews, meta-analyses, and editorials (Fig. 1B). After applying our exclusion criteria, we identified a total of 49 unique cohorts for analysis (Table 1). Focal cryotherapy comprised 21/49 (42.9%) cohorts [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31]. Focal HIFU comprised 20/49 (40.8%) of the cohorts [23, 32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50]. Focal IRE comprised 8/49 (16.3%) of the cohorts [51,52,53,54,55,56,57,58].
Evolution of patient inclusion criteria and selection tools over time
The patient inclusion criteria for FT among studies was highly heterogeneous, including a range of low-, intermediate-, and high-risk patients, as well as a large range of GGs. In all, only 29/49 (59.2%) of cohorts reported D’Amico/NCCN/CAPRA risk stratification, while 48/49 (98.0%) of cohorts reported ISUP GG compositions of their patients. Earlier cohorts were more likely to report the risk strata compositions of their cohorts. In the 2018–2012 time period, 4/4 (100%) of cohorts reported risk stratification; from 2013 to 2016, 7/7 (100%); from 2017 to 2020, 8/13 (61.5%); and from 2021 to 2024, 10/25 (40.0%).
Over time, we observed a gradual increase in the proportion of GG2-5 patients in the reported cohorts, whereas earlier cohorts generally included a higher proportion of GG1 patients, reflecting a change in the patient selection criteria for FT to target patients with clinically significant prostate cancer (Fig. 1C). In the 2008–2012 time period, 4 studies reported that the proportion of their cohort having csPCa ranged from 35.0 to 42.5%; from 2013 to 2016, 7 studies reported 0.0–57.2%; from 2017 to 2020, 13 studies 16.0–74.3%; and, from 2021 to 2024, 25 studies 66.3–85.3%.
A total of 38/49 (77.6%) of cohorts reported the use of mpMRI in the pre-diagnostic workup before FT. Later cohorts were more likely to utilize mpMRI compared to earlier cohorts. In the 2008–2012 time period, 0/4 (0%) studies reported utilizing mpMRI; from 2013 to 2016, 4/7 (57.1%); from 2017 to 2020, 12/13 (92.3%); and, from 2021 to 2024, 22/25 (88.0%) studies reported utilizing mpMRI. 27/49 (55.1%) of cohorts reported utilizing targeted biopsies while 26/49 (53.1%) cohorts reported using some form of systematic biopsy (12-core or mapping biopsy).
Evolution of treatment patterns over time
Overall, 14/49 (28.6%) of cohorts reported using hemi-ablation; 26/49 (53.1%), focal ablation; 1/49 (2.0%), partial gland ablation; 1/49 (2.0%), sectoral ablation; and, 6/49 (12.2%), various combinations. Over time, there was a greater proportion of cohorts utilizing more focal approaches and less hemi-ablations. In the 2008–2012 time period, 3/4 (75%) of cohorts reported utilizing hemi-ablation for FT; from 2013 to 2016, 4/7 (57.1%); from 2017 to 2020, 6/13 (46.2%); and, from 2021 to 2024 only 1/25 (4.0%).
During our evaluation period, cryotherapy was predominant in the earlier years, with an increasing proportion of later-reported cohorts comprising HIFU and IRE. In the 2008–2012 time period, 4/4 (100%) of cohorts reported using cryotherapy; from 2013 to 2016, 4/7 (57.1%) cryotherapy, 2/7 (28.6%) HIFU, and 1/7 (14.3%) IRE; from 2017 to 2020, 5/13 (38.4%) cryotherapy, 7/13 (53.8%) HIFU, and 1/13 (7.7%) IRE; and, from 2021 to 2024, 8/25 (32.0%) cryotherapy, 11/25 (44.0%) HIFU, and 6/25 (24.0%) IRE.
Oncological outcomes
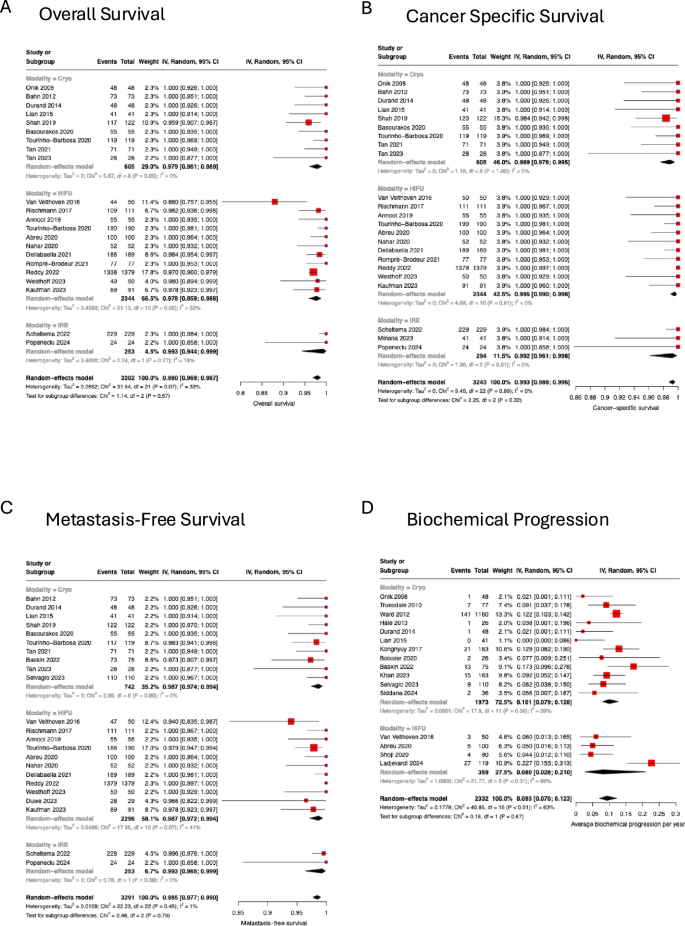
Median follow-up times were reported in 45/49 (91.8%) cohorts, with a median of 27.8 months and a mean of 28.4 months (range 6.0–63.0 months). Overall survival was reported by 22/49 (44.9%) cohorts with a pooled OS of 98.0% (95% CI 96.9–98.7, I2 = 33%, p = 0.07) (Fig. 2A). Cancer-specific survival was reported by 23/49 (46.9%) cohorts with a pooled CSS of 99.3% (95% CI 98.8–99.6, I2 = 0%, p = 0.99) (Fig. 2B). Metastasis-free survival was reported by 23/49 (46.9%) cohorts with a pooled MFS of 98.5% (95% CI I2 = 0%, p = 0.46) (Fig. 2C). There were no differences observed between different modalities for OS, MFS or CSS.
A Pooled overall survival; B pooled cancer-specific survival; C pooled metastasis-free survival, D pooled yearly biochemical progression rate.
Biochemical progression was reported in 15/49 (30.6%) cohorts. Of these, 12/15 (80.0%) utilized the Phoenix criteria, 2/15 (13.3%) used the ASTRO criteria, and 1/15 (6.7%) used a nadir + 0.5 definition. The average biochemical progression rate per year was 9.3% (95% CI 7.0–12.3, I2 = 63%, p < 0.01) (Fig. 2D). Again, there were no differences observed between different modalities.
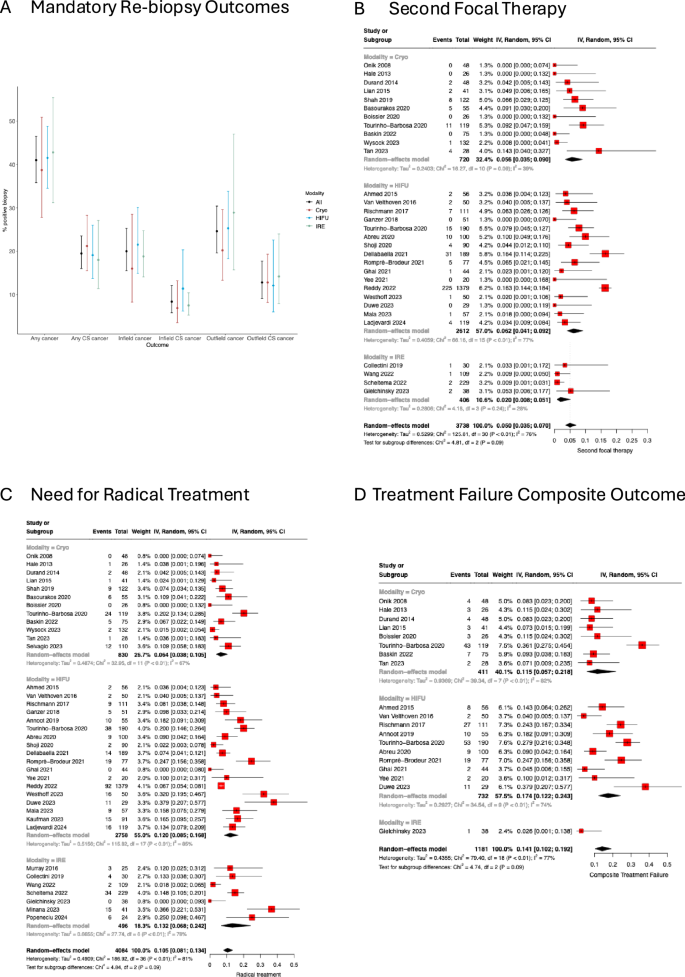
Repeat biopsy was mandated within 24 months in 36/49 (73.5%) cohorts and for cause in the remaining 13/49 (26.5%) cohorts. Among cohorts with mandated repeat biopsy in their follow-up protocol, the mean actual repeat biopsy rate was 84.5% (±18.8%), while for cohorts without mandated repeat biopsy, the biopsy rate was 28.7% (±16.0%). Among the 36 mandated repeat biopsy cohorts, 26/36 (72.2%) reported a pooled overall, any cancer-positive biopsy rate of 44.6% (95% CI 38.7–50.6%, I2 = 86%, p < 0.01); 29/36 (80.6%) reported a pooled overall, clinically significant cancer positive biopsy rate of 22.2% (95% CI 17.5–27.7%, I2 = 89%, p < 0.01); 25/36 (69.4%) reported a pooled infield, any cancer positive biopsy rate of 21.4% (16.9–26.8%, I2 = 79%, p < 0.01); 24/36 (66.7%) reported a pooled infield, clinically significant cancer positive biopsy rate of 8.9% (95% CI 6.2–12.5%, I2 = 70%, p < 0.01); 25/36 (69.4%) reported a pooled outfield, any cancer positive biopsy rate of 26.5% (95% CI 21.6–32.0%, I2 = 77%, p < 0.01); and, 22/36 (61.1%) reported a pooled outfield, clinically significant cancer positive biopsy rate of 12.3% (95% CI 8.7–17.1%, I2 = 77%, p < 0.01). These findings are summarized in Fig. 3A, and detailed in Supplementary Figs. 1–24. We did not observe a clinically significant difference in positive repeat biopsy rates between the focal cryotherapy, HIFU, or IRE cohorts. Notably, there was high heterogeneity across the cohorts, and as such, a formal comparison of different modalities was not performed.
A Error bar plot of the rate of positive biopsy in any location, infield or outfield, subdivided into any cancer versus clinically significant (CS) cancer, stratified by modality; B pooled rate of second focal therapy (FT); C pooled rate of salvage radical therapy (both radical prostatectomy and radiation therapy); D pooled rate of composite treatment failure (salvage whole gland ablation, salvage radical therapy, salvage hormonal therapy, transition to watchful waiting).
The rate of requiring a second FT was reported in 31/49 (63.3%) cohorts with a pooled incidence of 5.0% (95% CI 3.5–7.0%, I2 = 76%, p < 0.01) (Fig. 3B). The rate of salvage radical treatment with prostatectomy or radiation therapy was reported in 37/49 (75.5%) cohorts with a pooled incidence of 10.5% (95% CI 8.1–13.4%, I2 = 81%, p < 0.01) (Fig. 3C). The rate of salvage whole gland ablation was reported in 27/49 (55.1%) cohorts with a pooled incidence of 2.1% (95% CI 1.3–3.4%, I2 = 54%, p < 0.01) (Supplementary Fig. 25). The use of salvage hormonal therapy was reported in 23/49 (46.9%) cohorts, with a pooled incidence of 3.4% (95% CI 2.5–4.6%, I2 = 20%, p = 0.19) (Supplementary Fig. 26). Transition to watchful waiting was reported in 22/49 (44.9%) cohorts, with a pooled incidence of 3.0% (95% CI 1.7–5.3%, I2 = 66%, p < 0.01) (supplementary Fig. 27). Due to varying definitions of treatment failure, we adopted a composite outcome of radical or whole gland treatment, use of hormonal therapy or a transition to watchful waiting, not including a second FT. Using this definition, 19/49 (38.8%) cohorts had a pooled failure rate of 14.1% (95% CI 10.2–19.2%, I2 = 77%, p < 0.01) (Fig. 3D).
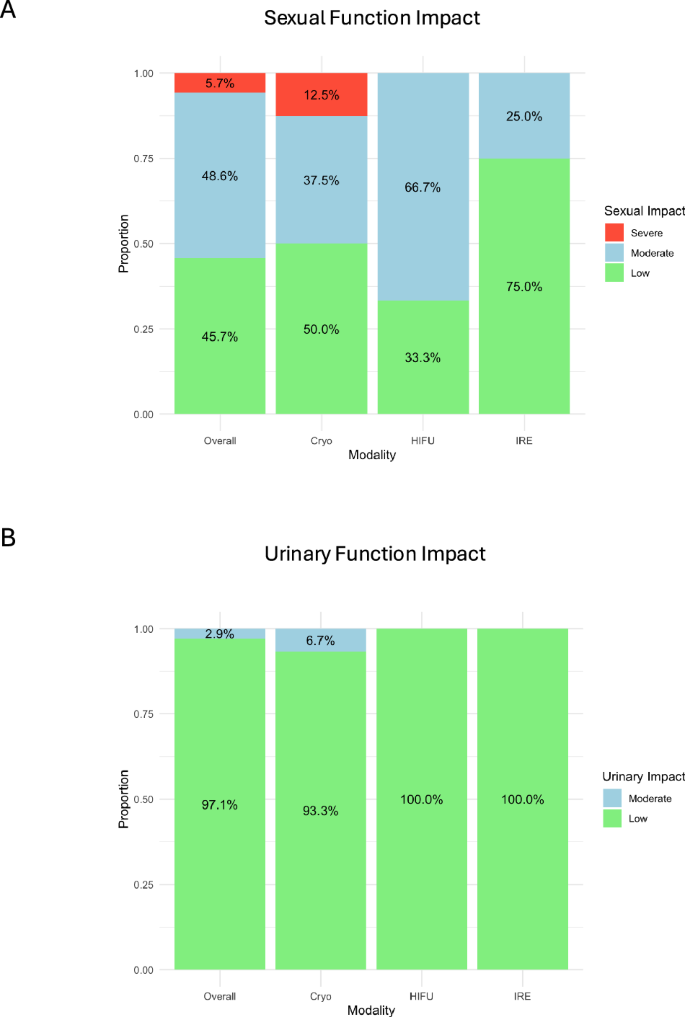
Sexual and urinary function
Of 35/49 (71.4%) cohorts reporting sexual function outcomes, 23/35 (65.7%) used patient-reported outcome measures (17 utilized IIEF/SHIM; 5, EPIC; 1, EORTC QLQC-28), and 12/35 (34.3%) used physician-reported measures (10 reported the ability to penetrate; 2, the need for medications/assistance for sexual intercourse). We summarized the impact on sexual function was categorized as low, moderate, or severe.
Overall, 16/35 (45.7%) cohorts reported a low impact of FT on sexual function, 17/35 (48.6%) reported a moderate impact, and 2/35 (5.7%) a severe impact (Fig. 4A). No significant differences in sexual impact were noted between the different modalities.
A Sexual function impact: low (<10%), medium (10–30%), and high (>30%), stratified by modality; B Urinary function impact: low (<10%), medium (10–30%) and high (>30%), stratified by modality.
Of 34/49 (69.4%) cohorts reporting urinary function outcomes, 21/34 (61.8%) used patient-reported outcome measures (12 reported using the AUA symptom index; 7, EPIC; 1, the ICIQ-UI, and 1, the EORTC QLQC-28), while 13/34 (38.2%) reported pad use only. The impact on urinary function was again categorized as low, moderate, or severe.
Overall, 33/34 (97.1%) cohorts reported a low impact of FT on urinary function, 1/34 (2.9%) cohort reported a moderate impact, and none of the cohorts reported a severe impact on urinary function (Fig. 4B). No significant difference on urinary function impact was noted between different FT modalities.
Discussion
Where are the randomized controlled trials (RCTs) for FT?
Randomized evidence for the oncological efficacy of FT compared to standard radical treatments remains lacking. One reason being that experience in recruitment for RCTs has not been encouraging: while the Partial ablation versus prostatectomy (PART) trial showed initial feasibility in a 2018 report, the current recruitment at the time of writing shows only 42 of the targeted 800 patients recruited [59, 60]. Similarly, IP4-Chronos concluded that it is not feasible to do a randomized study in FT because of strong patient preferences when it comes to prostate cancer treatment [61]. Perhaps with these experiences in consideration, rather than evaluating oncological outcomes as the primary endpoint, the latest proposed randomized trial comparing FT to standard radical treatment, the Prostate Cancer IRE study (PRIS), will evaluate quality of life as the primary endpoint as this requires a smaller sample size [62].
What can we learn from current observational data?
Here, we evaluated 49 unique cohorts of men undergoing FT for prostate cancer. The studies were highly heterogeneous in terms of patient inclusion, oncological/functional outcomes, and follow-up surveillance measures. For instance, OS, CSS, and MFS were reported only in half the cohorts, though the heterogeneity was low. The pooled OS was 98.0%, CSS 99.3%, and MFS 98.5%. Biochemical progression was generally reported in older cohorts, but heterogeneity was high likely due to different follow-up times and progression criteria. A pooled yearly biochemical progression rate of 9.3% was reported. This was the main trigger for for-cause biopsy for which the pooled rate was 38.7%. Among the 73.5% of studies with mandated biopsies within 24 months, the average biopsy compliance rate was 84.5% and csPCa detection rate was 22.2% overall, 8.9% infield, and 12.3% outfield. Again, there was significant heterogeneity in these results, contributed by the fact that many studies omitted the location of recurrences (infield/outfield) or the grade of the recurrences (GG1 vs ≥2). The pooled rate of repeat with FT was 5.0%, radical treatment 10.5%, and the the pooled yearly composite treatment failure rate, which comprises the use of radical treatment, whole-gland ablation, hormonal therapy, or transition to watchful waiting was 14.1% per year. This appears to be a fairly high rate of failure, but it is notable that these tended to be reported in studies mandating repeat biopsy with a fairly short follow-up time, so they may represent failures detected early on. The longitudinal effect of FT over time remains to be seen with regards to treatment failure as an outcome.
The outcomes of FT are highly dependent on patient selection. The more aggressive tumors included, the higher the risk of recurrence post-FT. We noted an evolving trend including a greater proportion of csPCa in the patient cohorts receiving treatment. This is in accordance with findings from expert consensus over time in which recommendations for patient selection for FT have also evolved. In 2010 and 2012 consensuses, de la Rosette et al and Ahmed et al respectively found that experts recommended eradication of all cancer as the goal of FT [63, 64]. In 2015 and 2017 consensuses, Donaldson et al and Tay et al respectively found that experts recommended eradication of csPCa [65, 66]. In this particular analysis, we did not have enough data to analyze the impact of the proportion of csPCa included with regards to the oncological outcomes. Another important question is that of untreated GG1 cancer whilst the csPCa lesion is being treated. In the 2015 and 2017 consensuses, experts agreed that GG1 cancer could be left untreated during FT to a csPCa lesion [65, 66]. We found that there was very limited reporting on the incidence and fate of untreated low-grade cancer foci in the various cohorts.
We also observed that ablation patterns changed over time with more focal/targeted ablations being done compared to hemi-ablations. This trend follows that of the rapid adoption of imaging and targeted biopsy over the last decade. With the ability to better identify and localize csPCa, there is greater confidence in treating just the area containing the aggressive tumor for true FT rather than hemi-ablation [67]. In this regard, Zhang et al. demonstrated in a randomized clinical trial that extending ablation to the entire hemi-gland added no advantage to focal ablation using IRE [58]. However, given the small but significant rates of cancer persistence in or around the treatment zone, there is still a necessity to ensure adequate margins around the lesion, and an adequate delivery of ablative energy, regardless of FT modality within the area to be treated itself [68, 69].
We did not observe significant differences between the three established FT modalities in oncological or functional outcome. This could reflect increased familiarity with each modality by the treating physicians over time leading to technical measures to improve outcome such as double freeze-thaw cycles for cryotherapy and double-tap for HIFU. Sivaraman et al proposed that anterior tumors are better treated using cryotherapy whereas posterior tumors are better treated with HIFU, in a concept termed as the a la carte model [70]. Nevertheless, this strategy has never been validated. We observed that the vast majority of cohorts reported only using a single FT modality, with the exception of the UK group which reported an 80% of their focal cryotherapy series comprising of anterior tumors, and the Montsouris group where the a la carte model originated [20, 23]. In the few (12/49, 24.5%) cohorts reporting on tumor location, the proportion of anterior tumors in the cryotherapy cohorts were not observably different than that of the HIFU cohorts. It may be the case that even in academic centers, expertise in a single FT modality is more feasible than having multiple competing modalities that may take more financial resources and time to master.
Limitations of this review
This systematic review must be interpreted with several limitations in mind. First, we included only single-arm cohorts reporting cryotherapy, HIFU, and IRE FT. We did not include outcomes of newer modalities which have been evaluated in randomized or non-randomized comparative studies. Second, the heterogeneity among the included studies was high for aspects ranging from patient inclusion criteria to outcomes measures and follow-up protocol. This limits the generalization of our findings. Third, the follow-up time after FT remains short. The overall median follow-up time of the included cohorts was 25.8 months (range 6–63). This limits evaluation of OS, CSS, and MFS. Biopsy outcomes can be a surrogate measure but are by no means definitive. Fourth, we evaluated only the specific domains of sexual and urinary function. These were the most reported functional domains and it would not be possible to meaningfully assess other functions.
Where does this leave us with focal therapy in 2024 and how do we proceed from here?
Based on our findings, it is clear that significant work remains to be done to define the role of FT in the treatment of prostate cancer. Major guidelines still recommend that FT, even with established modalities, be performed at least within a prospective registry [1]. Until the field is able to provide a clear patient-selection criteria and demonstrate definitive long-term oncological outcomes, these recommendations are likely to stand.
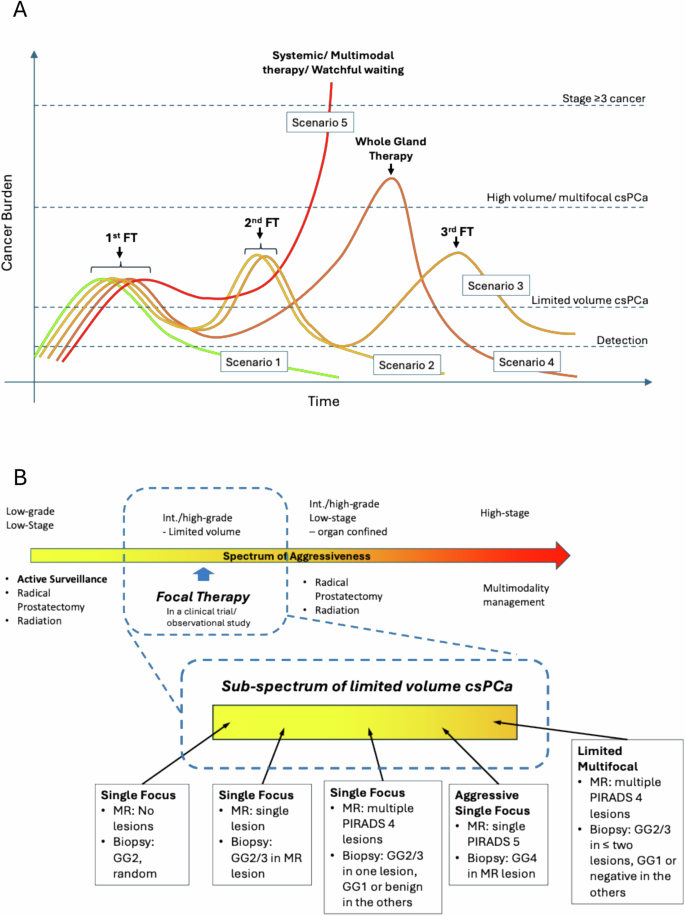
Overall, cancer-specific and metastasis-free survival remain the ideal standards for which FT can be compared to standard radical therapy in an RCT. However, even with sufficient dedication to achieve a long enough follow-up, our experience with RCTs suggest that the cross-over rate among treatments will be high due to patient preference, thus impacting intention to treat analysis. Other proposed outcomes such as various determinations of local, biochemical, or treatment progression (repeat or salvage) would simply be incomparable between standard radical therapy and FT (Fig. 5A). When well recorded, however, they could be suitable surrogate oncological outcomes in prospective FT cohorts.
A Potential pathways of prostate cancer progression after focal therapy (FT); B pre-FT cancer patterns or “phenotypes” that may impact on interpretation of post-FT imaging/biopsy/progression outcomes.
Is biopsy still necessary after FT? After all, breast surgeons do not do breast biopsies after lumpectomy, and we do not routinely biopsy the kidney after ablation or partial nephrectomy for kidney cancer [71]. An imaging-only approach may be possible in a high-volume FT center with expert radiologists, but many community radiologists may not be experienced in reading post-FT MRIs [72, 73]. Lebastchi et al reported that majority of experts favored repeat biopsy 6–12 months after FT [74]. An early repeat biopsy also serves as a quality indicator of the ablation, and allows for early repeat ablation, or fallback to radical treatment, to ensure adequate oncological care.
What is certainly necessary, is detailed standardized reporting of pre- and post-FT imaging and biopsy. In this systematic review, of cohorts mandating repeat post-FT biopsy, only 27.8% reported complete outcomes (infield, outfield, cs- and non-csPCa). We would argue that the post-FT imaging and biopsy outcomes can only be well understood in the context of the pre-FT cancer pattern. For example, a patient with a unifocal PIRADS 4 lesion, with csPCa in corroborating targeted cores and a negative systematic biopsy would likely do well with FT, while another patient with multiple PIRADS 4 lesions, in which one harbors GG2 cancer treated with FT, and the others have low volume GG1 cancer on surveillance might be at higher risk of needing secondary FT or whole-gland treatment. The pre-FT cancer pattern could possibly be classified into several “phenotypes” which represent different scenarios (Fig. 5B). Identifying and reporting these disparate patterns could help to put the post-FT imaging/biopsy outcomes in context and thus better define oncological outcomes after FT.
As advanced imaging techniques, like PSMA-PET, multiparametric ultrasound, and genomic biomarkers continue to evolve, the necessity for standardized, detailed reporting grows. This ensures accurate identification and definition of the aggressive cancer for treatment. Future work should thus focus on standardized, accurate, and detailed reporting of the patients treated, including both treated and untreated lesions, their imaging and pathological characteristics, treatment zones, follow-up protocols, and detailed failure data.
While other excellent systematic reviews on FT have been done in the past, they have been of a broad scope focused on all available modalities [8, 75]. This systematic review done on established FT modalities is a more recent update (25 FT cohorts have been reported/updated in the last 4 years alone), incorporates what long-term data is available, provides an overview of where we are now with more “mature” oncological and functional outcomes, providing an overview of the gaps in reporting that we need in order to make the next systematic review a definitive one.
Conclusion
There is an increased uptake of focal therapy over time with a greater proportion of patients with csPCa rather than low-grade cancer undergoing treatment. FT with cryotherapy, HIFU, or IRE is associated with good short-intermediate term oncological and functional outcomes. However, outcome reporting is heterogeneous and often incomplete. Longer-term follow-up and standardized reporting are required to better define and report FT outcomes.
Data availability
Data are available for bona fide researchers who request it from the authors.
References
Cornford P, van den Bergh RCN, Briers E, Van den Broeck T, Brunckhorst O, Darraugh J, et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG guidelines on prostate cancer-2024 update. Part I: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2024;86:148–63.
Wallis CJ, Herschorn S, Saskin R, Su J, Klotz LH, Chang M, et al. Complications after radical prostatectomy or radiotherapy for prostate cancer: results of a population-based, propensity score-matched analysis. Urology. 2015;85:621–7.
Popiolek M, Rider JR, Andrén O, Andersson S-O, Holmberg L, Adami H-O, et al. Natural history of early, localized prostate cancer: a final report from three decades of follow-up. Eur Urol. 2013;63:428–35.
Klotz L, Vesprini D, Sethukavalan P, Jethava V, Zhang L, Jain S, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol. 2015;33:272–7.
Tay KJ. Prostate focal therapy: the rule or exception? Curr Opin Urol. 2018;28:512–21.
Ahmed HU, El-Shater Bosaily A, Brown LC, Gabe R, Kaplan R, Parmar MK, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet. 2017;389:815–22.
Siddiqui MM, Rais-Bahrami S, Turkbey B, George AK, Rothwax J, Shakir N, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA. 2015;313:390–7.
Hopstaken JS, Bomers JGR, Sedelaar MJP, Valerio M, Futterer JJ, Rovers MM. An updated systematic review on focal therapy in localized prostate cancer: what has changed over the past 5 years? Eur Urol. 2022;81:5–33.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2024. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
Onik G, Vaughan D, Lotenfoe R, Dineen M, Brady J. The “male lumpectomy”: focal therapy for prostate cancer using cryoablation results in 48 patients with at least 2-year follow-up. Urol Oncol. 2008;26:500–5.
Truesdale MD, Cheetham PJ, Hruby GW, Wenske S, Conforto AK, Cooper AB, et al. An evaluation of patient selection criteria on predicting progression-free survival after primary focal unilateral nerve-sparing cryoablation for prostate cancer: recommendations for follow up. Cancer J. 2010;16:544–9.
Bahn D, de Castro Abreu AL, Gill IS, Hung AJ, Silverman P, Gross ME, et al. Focal cryotherapy for clinically unilateral, low-intermediate risk prostate cancer in 73 men with a median follow-up of 3.7 years. Eur Urol. 2012;62:55–63.
Ward JF, Jones JS. Focal cryotherapy for localized prostate cancer: a report from the national Cryo On-Line Database (COLD) Registry. BJU Int. 2012;109:1648–54.
Hale Z, Miyake M, Palacios DA, Rosser CJ. Focal cryosurgical ablation of the prostate: a single institute’s perspective. BMC Urol. 2013;13:2.
Barqawi AB, Stoimenova D, Krughoff K, Eid K, O’Donnell C, Phillips JM, et al. Targeted focal therapy for the management of organ confined prostate cancer. J Urol. 2014;192:749–53.
Durand M, Barret E, Galiano M, Rozet F, Sanchez-Salas R, Ahallal Y, et al. Focal cryoablation: a treatment option for unilateral low-risk prostate cancer. BJU Int. 2014;113:56–64.
Lian H, Zhuang J, Yang R, Qu F, Wang W, Lin T, et al. Focal cryoablation for unilateral low-intermediate-risk prostate cancer: 63-month mean follow-up results of 41 patients. Int Urol Nephrol. 2016;48:85–90.
Kongnyuy M, Lipsky MJ, Islam S, Robins DJ, Hager S, Halpern DM, et al. Predictors of biochemical recurrence after primary focal cryosurgery (hemiablation) for localized prostate cancer: a multi-institutional analytic comparison of Phoenix and Stuttgart criteria. Urol Oncol. 2017;35:530 e15–19.
Shah TT, Peters M, Eldred-Evans D, Miah S, Yap T, Faure-Walker NA, et al. Early-medium-term outcomes of primary focal cryotherapy to treat nonmetastatic clinically significant prostate cancer from a prospective multicentre registry. Eur Urol. 2019;76:98–105.
Basourakos SP, Al Hussein Al Awamlh B, Bianco FJ, Patel NA, Laviana A, Margolis DJ, et al. Feasibility of in-office MRI-targeted partial gland cryoablation for prostate cancer: an IDEAL stage 2A study. BMJ Surg Interv Health Technol. 2020;2:e000056.
Boissier R, Sanguedolce F, Territo A, Vanacore D, Martinez C, Regis F, et al. Whole and hemi-gland cryoablation for primary localized prostate cancer: short and medium-term oncological and functional outcomes. Actas Urol Esp. 2020;44:172–8.
Tourinho-Barbosa RR, Sanchez-Salas R, Claros OR, Collura-Merlier S, Bakavicius A, Carneiro A, et al. Focal therapy for localized prostate cancer with either high intensity focused ultrasound or cryoablation: a single institution experience. J Urol. 2020;203:320–30.
Tan WP, Chang A, Sze C, Polascik TJ. Oncological and functional outcomes of patients undergoing individualized partial gland cryoablation of the prostate: a single-institution experience. J Endourol 2021;35:1290–9.
Baskin A, Charondo LB, Balakrishnan A, Cowan JE, Cooperberg MR, Carroll PR, et al. Medium-term outcomes of focal cryoablation for intermediate and high-risk prostate cancer: MRI and PSA are not predictive of residual or recurrent disease. Urol Oncol. 2022;40:451 e15–20.
Aker MN, Brisbane WG, Kwan L, Gonzalez S, Priester AM, Kinnaird A, et al. Cryotherapy for partial gland ablation of prostate cancer: oncologic and safety outcomes. Cancer Med. 2023;12:9351–62.
Tan YG, Law YM, Ngo NT, Khor LY, Tan PH, Ong EHW, et al. Patient-reported functional outcomes and oncological control after primary focal cryotherapy for clinically significant prostate cancer: a Phase II mandatory biopsy-monitored study. Prostate. 2023;83:781–91.
Wysock JS, Rapoport E, Hernandez H, Gogaj R, Lepor H. Biopsy assessment of oncologic control 3 years following primary partial gland cryoablation: a prospective cohort study of men with intermediate-risk prostate cancer. J Urol. 2023;210:454–64.
Khan A, Khan AU, Siref L, Feloney M. Focal cryoablation of the prostate: primary treatment in 163 patients with localized prostate cancer. Cureus. 2023;15:e37172.
Selvaggio O, Finati M, Falagario UG, Silecchia G, Recchia M, Checchia AA, et al. Treatment of localized prostate cancer in elderly patients: the role of partial cryoablation. Int Urol Nephrol. 2023;55:1125–32.
Sidana A, Tayebi S, Blank F, Lama DJ, Meyer M, Saeed Y, et al. Magnetic resonance imaging-ultrasound fusion guided focal cryoablation for men with intermediate-risk prostate cancer. Urol Oncol. 2024;42:158.e1–10.
Ahmed HU, Dickinson L, Charman S, Weir S, McCartan N, Hindley RG, et al. Focal ablation targeted to the index lesion in multifocal localised prostate cancer: a prospective development study. Eur Urol. 2015;68:927–36.
van Velthoven R, Aoun F, Marcelis Q, Albisinni S, Zanaty M, Lemort M, et al. A prospective clinical trial of HIFU hemiablation for clinically localized prostate cancer. Prostate Cancer Prostatic Dis. 2016;19:79–83.
Rischmann P, Gelet A, Riche B, Villers A, Pasticier G, Bondil P, et al. Focal high intensity focused ultrasound of unilateral localized prostate cancer: a prospective multicentric hemiablation study of 111 patients. Eur Urol. 2017;71:267–73.
Ganzer R, Hadaschik B, Pahernik S, Koch D, Baumunk D, Kuru T, et al. Prospective multicenter phase II study on focal therapy (hemiablation) of the prostate with high intensity focused ultrasound. J Urol. 2018;199:983–9.
Annoot A, Olivier J, Valtille P, Deken V, Leroy X, Puech P, et al. Extra-target low-risk prostate cancer: implications for focal high-intensity focused ultrasound of clinically significant prostate cancer. World J Urol. 2019;37:261–8.
Abreu AL, Peretsman S, Iwata A, Shakir A, Iwata T, Brooks J, et al. High intensity focused ultrasound hemigland ablation for prostate cancer: initial outcomes of a United States series. J Urol. 2020;204:741–7.
Nahar B, Bhat A, Reis IM, Soodana-Prakash N, Becerra MF, Lopategui D, et al. Prospective evaluation of focal high intensity focused ultrasound for localized prostate cancer. J Urol. 2020;204:483–9.
Shoji S, Hiraiwa S, Uemura K, Nitta M, Hasegawa M, Kawamura Y, et al. Focal therapy with high-intensity focused ultrasound for the localized prostate cancer for Asian based on the localization with MRI-TRUS fusion image-guided transperineal biopsy and 12-cores transperineal systematic biopsy: prospective analysis of oncological and functional outcomes. Int J Clin Oncol. 2020;25:1844–53.
Dellabella M, Branchi A, Di Rosa M, Pucci M, Gasparri L, Claudini R, et al. Oncological and functional outcome after partial prostate HIFU ablation with Focal-One((R)): a prospective single-center study. Prostate Cancer Prostatic Dis. 2021;24:1189–97.
Ghai S, Finelli A, Corr K, Chan R, Jokhu S, Li X, et al. MRI-guided focused ultrasound ablation for localized intermediate-risk prostate cancer: early results of a phase II trial. Radiology. 2021;298:695–703.
Rompre-Brodeur A, Marcq G, Tholomier C, Fugaru I, Loutochin O, Anidjar M, et al. Role of systematic control biopsies following partial gland ablation with high-intensity focused ultrasound for clinically significant prostate cancer. J Urol. 2021;206:1177–83.
Yee CH, Chiu PK, Teoh JY, Ng CF, Chan CK, Hou SM. High-intensity focused ultrasound (HIFU) focal therapy for localized prostate cancer with MRI-US fusion platform. Adv Urol. 2021;2021:7157973.
Reddy D, Peters M, Shah TT, van Son M, Tanaka MB, Huber PM, et al. Cancer control outcomes following focal therapy using high-intensity focused ultrasound in 1379 men with nonmetastatic prostate cancer: a multi-institute 15-year experience. Eur Urol. 2022;81:407–13.
Duwe G, Boehm K, Haack M, Sparwasser P, Brandt MP, Mager R, et al. Single-center, prospective phase 2 trial of high-intensity focused ultrasound (HIFU) in patients with unilateral localized prostate cancer: good functional results but oncologically not as safe as expected. World J Urol. 2023;41:1293–9.
Mala KS, Plage H, Modl L, Hofbauer S, Friedersdorff F, Schostak M, et al. Follow-up of men who have undergone focal therapy for prostate cancer with HIFU-a real-world experience. J Clin Med. 2023;12:7089.
Westhoff N, Ernst R, Kowalewski KF, Derigs F, Neuberger M, Norenberg D, et al. Medium-term oncological efficacy and patient-reported outcomes after focal high-intensity focused ultrasound: the FOXPRO trial. Eur Urol Focus. 2023;9:283–90.
Kaufmann B, Raess E, Schmid FA, Bieri U, Scherer TP, Elleisy M, et al. Focal therapy with high-intensity focused ultrasound for prostate cancer: 3-year outcomes from a prospective trial. BJU Int. 2024;133:413–24.
Ladjevardi S, Ebner A, Femic A, Huebner NA, Shariat SF, Kraler S, et al. Focal high-intensity focused ultrasound therapy for localized prostate cancer: an interim analysis of the multinational FASST study. Eur J Clin Investig. 2024;54:e14192.
Ehdaie B, Tempany CM, Holland F, Sjoberg DD, Kibel AS, Trinh QD, et al. MRI-guided focused ultrasound focal therapy for patients with intermediate-risk prostate cancer: a phase 2b, multicentre study. Lancet Oncol. 2022;23:910–8.
Murray KS, Ehdaie B, Musser J, Mashni J, Srimathveeravalli G, Durack JC, et al. Pilot study to assess safety and clinical outcomes of irreversible electroporation for partial gland ablation in men with prostate cancer. J Urol. 2016;196:883–90.
Collettini F, Enders J, Stephan C, Fischer T, Baur ADJ, Penzkofer T, et al. Image-guided irreversible electroporation of localized prostate cancer: functional and oncologic outcomes. Radiology. 2019;292:250–7.
Wang H, Xue W, Yan W, Yin L, Dong B, He B, et al. Extended focal ablation of localized prostate cancer with high-frequency irreversible electroporation: a nonrandomized controlled trial. JAMA Surg. 2022;157:693–700.
Gielchinsky I, Lev-Cohain N. Focal irreversible electroporation for localized prostate cancer - oncological and safety outcomes using mpMRI and transperineal biopsy follow-up. Res Rep Urol. 2023;15:27–35.
Minana Lopez B, Andres Boville G, Barbas Bernardos G, Ancizu Marckert X, Torres Roca M, Labairu Huerta L, et al. Focal therapy of prostate cancer index lesion with irreversible electroporation. a prospective study with a median follow-up of 3 years. J Urol. 2023;209:261–70.
Scheltema MJ, Geboers B, Blazevski A, Doan P, Katelaris A, Agrawal S, et al. Median 5-year outcomes of primary focal irreversible electroporation for localised prostate cancer. BJU Int. 2023;131:6–13.
Popeneciu IV, Mohr MN, Strauss A, Leitsmann C, Trojan L, Reichert M. Personalized treatment strategy in “low-risk prostate cancer active surveillance candidates” using irreversible electroporation: prospective evaluation of feasibility, morbidity, functional and oncological outcomes. World J Mens Health. 2024;42:821–9
Zhang K, Teoh J, Laguna P, Dominguez-Escrig J, Barret E, Ramon-Borja JC, et al. Effect of focal vs extended irreversible electroporation for the ablation of localized low- or intermediate-risk prostate cancer on early oncological control: a randomized clinical trial. JAMA Surg. 2023;158:343–9.
Hamdy FC, Elliott D, le Conte S, Davies LC, Burns RM, Thomson C, et al. Partial ablation versus radical prostatectomy in intermediate-risk prostate cancer: the PART feasibility RCT. Health Technol Assess. 2018;22:1–96.
PART Trial Website. 2024.
Reddy D, Dudderidge T, Shah T, McCracken S, Arya M, Fiorentino F, et al. Comparative healthcare research outcomes of novel Surgery in prostate cancer (IP4-CHRONOS): Pilot RCT assessing feasibility of randomization for focal therapy in localized prostate cancer. J Clin Oncol. 2022;40:5086.
Lantz A, Nordlund P, Falagario U, Jäderling F, Özbek O, Clements M, et al. Prostate cancer IRE study (PRIS): a randomized controlled trial comparing focal therapy to radical treatment in localized prostate cancer. Eur Urol Open Sci. 2023;51:89–94.
de la Rosette J, Ahmed H, Barentsz J, Johansen TB, Brausi M, Emberton M, et al. Focal therapy in prostate cancer-report from a consensus panel. J Endourol. 2010;24:775–80.
Ahmed HU, Akin O, Coleman JA, Crane S, Emberton M, Goldenberg L, et al. Transatlantic consensus group on active surveillance and focal therapy for prostate cancer. BJU Int. 2012;109:1636–47.
Donaldson IA, Alonzi R, Barratt D, Barret E, Berge V, Bott S, et al. Focal therapy: patients, interventions, and outcomes-a report from a consensus meeting. Eur Urol. 2015;67:771–7.
Tay KJ, Scheltema MJ, Ahmed HU, Barret E, Coleman JA, Dominguez-Escrig J, et al. Patient selection for prostate focal therapy in the era of active surveillance: an International Delphi Consensus Project. Prostate Cancer Prostatic Dis. 2017;20:294–9.
Scheltema MJ, Tay KJ, Postema AW, de Bruin DM, Feller J, Futterer JJ, et al. Utilization of multiparametric prostate magnetic resonance imaging in clinical practice and focal therapy: report from a Delphi consensus project. World J Urol. 2017;35:695–701.
Brisbane WG, Priester AM, Ballon J, Kwan L, Delfin MK, Felker ER, et al. Targeted prostate biopsy: umbra, penumbra, and value of perilesional sampling. Eur Urol. 2022;82:303–10.
Aslim EJ, Law YXT, Fook-Chong SMC, Ho HSS, Yuen JSP, Lau WKO, et al. Defining prostate cancer size and treatment margin for focal therapy: does intralesional heterogeneity impact the performance of multiparametric MRI? BJU Int. 2021;128:178–86.
Sivaraman A, Barret E. Focal therapy for prostate cancer: an “A la Carte” approach. Eur Urol. 2016;69:973–5.
Labbate CV, Klotz L, Morrow M, Cooperberg M, Esserman L, Eggener SE. Focal therapy for prostate cancer: evolutionary parallels to breast cancer treatment. J Urol. 2023;209:49–57.
Giganti F, Dickinson L, Orczyk C, Haider A, Freeman A, Emberton M, et al. Prostate imaging after focal ablation (PI-FAB): a proposal for a scoring system for multiparametric MRI of the prostate after focal therapy. Eur Urol Oncol. 2023;6:629–34.
Velaga J, Tay KJ, Hang G, Tan YG, Yuen JS, Chua M, et al. Surveillance one year post focal cryotherapy for clinically significant prostate cancer using mpMRI and PIRADS v2.1: an initial experience from a prospective phase II mandatory biopsy study. Eur J Radio Open. 2023;11:100529.
Lebastchi AH, George AK, Polascik TJ, Coleman J, de la Rosette J, Turkbey B, et al. Standardized nomenclature and surveillance methodologies after focal therapy and partial gland ablation for localized prostate cancer: an international multidisciplinary consensus. Eur Urol. 2020;78:371–8.
Valerio M, Ahmed HU, Emberton M, Lawrentschuk N, Lazzeri M, Montironi R, et al. The role of focal therapy in the management of localised prostate cancer: a systematic review. Eur Urol. 2014;66:732–51.
Funding
National Medical Research Council Singapore, Grant/Award Numbers: TA20nov-0011.
Ethics declarations
Competing interests
KJT reports personal fees and non-financial support from Boston Scientific; LRS and JLDE have consulted for AngioDynamics; OU has received non-financial support from Boston Scientific. AB has consulted for EDAP; The remaining authors have no conflicts to declare.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tay, K.J., Fong, K.Y., Stabile, A. et al. Established focal therapy—HIFU, IRE, or cryotherapy—where are we now?—a systematic review and meta-analysis. Prostate Cancer Prostatic Dis (2024). https://doi.org/10.1038/s41391-024-00911-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41391-024-00911-2
Subjects

Comments
Post a Comment