Pfizer's Cancer Drug Combo Improves Overall Survival in Late-stage Study
Pfizer's Cancer Drug Combo Improves Overall Survival in Late-stage Study
Short Summary for Patients
1. New Treatment Option:
- Pfizer has developed a new combination treatment for mCRPC.
- It combines two drugs: TALZENNA (talazoparib) and XTANDI (enzalutamide).
2. Who It's For:
- This treatment is for men with mCRPC, which means prostate cancer that:
- Has spread to other parts of the body
- No longer responds to hormone therapy or surgery to lower testosterone
3. How It Works:
- TALZENNA is a type of drug called a PARP inhibitor.
- XTANDI blocks the effects of male hormones on prostate cancer.
- Together, they attack cancer in two different ways.
4. Effectiveness:
- In clinical trials, this combination showed significant benefits:
- It helped patients live longer overall.
- It slowed down the progression of cancer.
- It works for many patients, but it's especially effective for those with certain genetic changes (mutations in genes like BRCA1, BRCA2, or CDK12).
5. Availability:
- This combination is approved for use in the United States and Europe.
- Your doctor can determine if it's right for you.
6. Important Considerations:
- Like all treatments, it can have side effects. Common ones include low blood cell counts and fatigue.
- Regular blood tests are needed to monitor your health during treatment.
- Discuss all potential benefits and risks with your healthcare provider.
7. Future Developments:
- Pfizer is working to make this treatment available to more patients with prostate cancer.
This new combination offers hope for many mCRPC patients, potentially extending life and slowing cancer progression. It's important to discuss this option with your oncologist to see if it's appropriate for your specific situation.
Summary of Talzenna+Xtandi Results
1. TALZENNA (talazoparib) in combination with XTANDI (enzalutamide):
- Approved for adult patients with metastatic castration-resistant prostate cancer (mCRPC) with homologous recombination repair (HRR) gene mutations.
- First and only PARP inhibitor plus androgen receptor pathway inhibitor (ARPI) combination to demonstrate statistically significant overall survival benefit in mCRPC patients.
- Approved in the U.S. in June 2023 and in the European Union in January 2024.
- Showed improvement in both radiographic progression-free survival (rPFS) and overall survival (OS) compared to XTANDI alone.
- Effective in patients regardless of HRR mutation status, though patients with BRCA1, BRCA2, or CDK12 gene alterations showed the most improvement.
2. XTANDI (enzalutamide) alone:
- An androgen receptor signaling inhibitor.
- Approved for various stages of prostate cancer, including metastatic castration-sensitive prostate cancer (mCSPC), metastatic castration-resistant prostate cancer (mCRPC), non-metastatic castration-resistant prostate cancer (nmCRPC), and nonmetastatic castration-sensitive prostate cancer (nmCSPC) with high-risk biochemical recurrence.
Key points:
- The TALZENNA + XTANDI combination significantly improved overall survival in mCRPC patients, both in the general population and in those with HRR gene mutations.
- This combination represents a new treatment option for patients with advanced prostate cancer, particularly those who have not responded to hormone therapy or surgical treatment to lower testosterone.
- The drugs have shown efficacy in treating prostate cancer that has spread to other parts of the body (metastatic) and is no longer responding to standard hormone-blocking treatments (castration-resistant).
- Pfizer is seeking to potentially expand the approved label for TALZENNA based on the positive results from the TALAPRO-2 trial.
These developments represent significant advancements in the treatment of advanced prostate cancer, offering new options for patients with limited treatment choices.
Clinical Impact of TALZENNA + XTANDI Combination in mCRPC
Overall Survival (OS)
- Statistically significant and clinically meaningful improvement in final OS analysis
- Benefit observed in all-comers (cohort 1) and HRR gene-mutated mCRPC patients (cohort 2)
- First PARP inhibitor + ARPI combination to show significant OS benefit in mCRPC
Radiographic Progression-Free Survival (rPFS)
- Maintained clinically meaningful improvement in rPFS from prior primary analysis
- In HRR-mutated cohort:
- Median rPFS not reached for combination vs. 14 months for XTANDI alone
- 55% reduction in risk of progression or death
Efficacy in Different Patient Populations
- Effective regardless of HRR mutation status
- Most benefit observed in patients with BRCA1, BRCA2, or CDK12 gene alterations
- Broader efficacy compared to other PARP inhibitors (e.g., niraparib + abiraterone)
Safety Profile
- Generally consistent with known safety profiles of individual medicines
- Common side effects: anemia, neutropenia, thrombocytopenia
- 39% of patients required red blood cell transfusion
Quality of Life
- Patients reported better quality of life for longer compared to XTANDI alone
Regulatory Status
- Approved in the US (June 2023) and EU (January 2024)
- Approved in over 35 countries globally
Potential for Expanded Use
- Results suggest possible benefit in broader mCRPC population
- Pfizer plans to share results with global health authorities for potential label expansion
Comparison to Other Treatments
- First PARP inhibitor to show significant survival benefit regardless of mutation status
- Potentially practice-changing efficacy according to lead investigator
Limitations and Considerations
- Long-term follow-up data still needed
- Balance of efficacy vs. side effects (e.g., higher transfusion rates) to be considered
- Optimal sequencing with other treatments yet to be determined
Questions to Ask Your Oncologist About mCRPC Treatment
- Q: Am I a candidate for the TALZENNA and XTANDI combination therapy?
A: Your oncologist will likely consider factors such as:
- Your overall health and ability to tolerate the treatment
- Whether you have specific genetic mutations (e.g., HRR genes like BRCA1/2)
- Your previous treatments and how you responded to them
- The extent and location of your cancer
- Q: How does this combination compare to other available treatments for my specific case?
A: Your oncologist should discuss:
- The potential benefits and risks compared to other options
- How it fits into the overall treatment strategy for your cancer
- Why they might recommend this over alternatives
- Q: What kind of improvement in survival and quality of life can I expect?
A: The oncologist might explain:
- Average improvements seen in clinical trials (e.g., median overall survival benefit)
- That individual results can vary
- Potential quality of life benefits and how they're measured
- Q: What are the most common and serious side effects of this treatment?
A: Expect a discussion about:
- Common side effects like anemia, fatigue, and nausea
- More serious but rarer side effects
- How these side effects are managed
- The likelihood of needing blood transfusions
- Q: How will we monitor the effectiveness of the treatment and any side effects?
A: Your oncologist should outline:
- Frequency of follow-up appointments
- Types of tests (e.g., blood tests, imaging scans) and their frequency
- Signs and symptoms you should watch for and report
- Q: Do I need genetic testing before starting this treatment, and if so, what kind?
A: The answer may include:
- Whether genetic testing is recommended or required
- What specific genes they're looking for (e.g., HRR mutations)
- How the results might affect your treatment plan
- Q: How long would I stay on this treatment, and what happens if it stops working?
A: Your oncologist should discuss:
- The typical duration of treatment
- How they determine if the treatment is working
- Potential next steps if the treatment becomes ineffective
- Q: Are there any lifestyle changes I should make while on this treatment?
A: Expect advice on:
- Diet and exercise recommendations
- Activities to avoid
- Strategies to manage fatigue or other side effects
- Q: Are there any clinical trials I should consider instead of or in addition to this treatment?
A: Your oncologist might:
- Discuss any relevant ongoing clinical trials
- Explain how participating in a trial might benefit you
- Outline the pros and cons of standard treatment vs. clinical trial participation
- Q: How will this treatment affect my day-to-day life?
A: The oncologist should provide information on:
- The treatment schedule and how it might impact your routine
- Potential limitations on work or other activities
- Strategies for maintaining quality of life during treatment
Remember, these are starting points for discussion. Don't hesitate to ask for clarification or more details on any aspect of your treatment plan.
(Reuters) - Pfizer said on Thursday a combination of its drugs, Talzenna and Xtandi, helped prolong the lives of patients with a type of advanced prostate cancer in a late-stage study.
The drug combination showed a significant improvement in the overall survival in patients with metastatic castration-resistant prostate cancer (mCRPC) regardless of the presence of a mutation, compared to Xtandi alone, Pfizer said.
Overall survival indicates the period of time patients lived after their diagnosis or the start of treatment.
mCRPC is an advanced stage of the disease where the cancer has spread to other parts of the body and is usually associated with poor prognosis. About 10%–20% of prostate cancer patients develop mCRPC within 5 to 7 years of diagnosis, according to Pfizer.
The Talzenna-Xtandi combination was approved by the U.S. Food and Drug Administration last year to treat mCRPC patients with a type of genetic mutation.
SUGGESTED FOR YOU
Pfizer said it plans to share the results with global health authorities to potentially update Talzenna's label.
The FDA had also approved AstraZeneca's Lynparza in combination with hormone therapy abiraterone last year to treat mCRPC patients with a type of mutation.
(Reporting by Mariam Sunny in Bengaluru; Editing by Shailesh Kuber)
Pfizer targets broad Talzenna approval in prostate cancer as trial meets patient survival goal
In prostate cancer, Pfizer’s Talzenna already holds the broadest FDA approval within the PARP inhibitor class. Now, with new data indicating the drug could prolong patients’ lives, the company is targeting an even wider label.
Adding Talzenna to Pfizer’s Astellas-partnered Xtandi significantly improved the life expectancy of patients with metastatic castration-resistant prostate cancer (mCPRC) regardless of the tumor’s mutation status, Pfizer said Thursday. The overall survival showing, from the final analysis of the phase 3 TALAPRO-2 trial, was statistically significant and clinically meaningful, the company said.
Armed with the updated data, Pfizer said it plans to share the results with global regulatory authorities to potential apply for a label expansion to cover a broad patient population with mCRPC.
In June 2023, the Talzenna-Xtandi combo received the FDA’s blessing to treat mCPRC that has homologous recombination repair (HRR) gene mutations.
That label is the broadest of an mCRPC indication among PARP inhibitors; AstraZeneca and Merck & Co.’s Lynparza—used in combination with Johnson & Johnson’s Zytiga—and J&J’s fixed-dose combo Akeega are only allowed in BRCA mutations, which are just part of the HRR alteration family.
With TALAPRO-2, Talzenna is now the first PARP inhibitor to be able to significantly improve survival in patients with mCRPC regardless of mutation status, Pfizer’s oncology chief development officer, Roger Dansey, M.D., said in a statement Thursday.
Neerja Agarawal, M.D., from the University of Utah and lead investigator for TALAPRO-2, said the results indicate potential “practice-changing efficacy,” according to a statement facilitated by Pfizer.
Related
Talzenna got its HRR-mutation nod based on TALAPRO-2 showing its addition to Xtandi could lower the risk of progression or death by 55% in those patients. At that time, the Talzenna-Xtandi combo also showed a 37% progression-free survival improvement versus Xtandi alone in a cohort that includes patients both with and without HRR mutations.
PARP inhibitors are expected to work best in tumors with BRCA mutations but not as much in other HRR mutations, with the least potential in those without HRR abnormalities.
In the phase 3 MAGNITUDE trial, J&J’s Akeega—a combination of niraparib (GSK’s Zejula) and Zytiga—didn’t show any benefit in mCRPC patients without HRR mutations. Lynparza’s mCRPC nod was also limited to BRCA mutations, because the FDA found those patients drove the benefit seen in the overall population enrolled in the phase 3 PROpel trial.
Related
Now, the key question for Pfizer’s updated TALAPRO-2 data is whether patients without HRR mutations saw any overall survival benefit. A Pfizer spokesperson said the company will share more details later.
During a previous interim analysis of TALAPRO-2, Talzenna showed a preliminary trend toward a 31% improvement in overall survival in HRR-mutated cases. At that time, only about one-fourth of the HRR-mutated population had passed away.
Talzenna is still a small asset in Pfizer’s oncology portfolio. The drug’s sales in the first half of 2024 were just $55 million, compared with $22 million during the same period last year. The company’s total oncology haul during the six months was $7.5 billion. Unlike Lynparza, Zejula and pharma&’s Rubraca, Talzenna doesn’t have an ovarian cancer indication, which has been the biggest field for PARP inhibitors.
Talazoparib Combo Approved for Metastatic Prostate Cancer
, by Shana Spindler
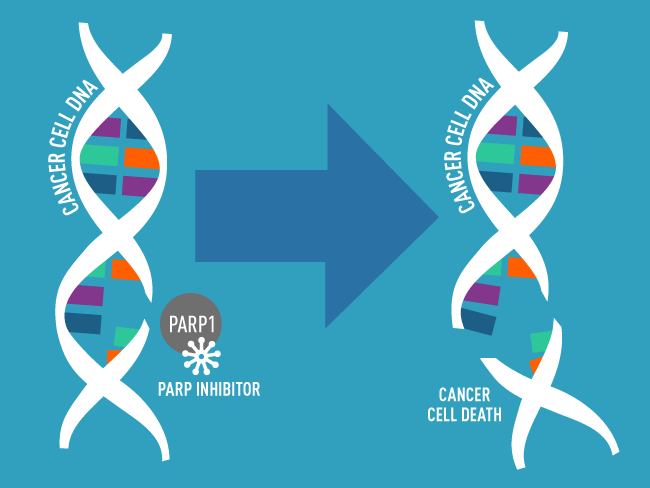
PARP inhibitors like talazoparib block PARP proteins from repairing damaged DNA, leading to further DNA damage and cell death.
Credit: National Cancer Institute
The Food and Drug Administration (FDA) has approved the combination of enzalutamide (Xtandi) with talazoparib (Talzenna) as an initial treatment for some people with metastatic castration-resistant prostate cancer. This is a form of prostate cancer that has spread from the prostate to other parts of the body and no longer responds to standard hormone-blocking treatments.
The talazoparib and enzalutamide combination is approved to treat people whose prostate cancer has an alteration in a specific group of genes involved in repairing damaged DNA. Talazoparib works by blocking the DNA repair activities of a protein called PARP, which in combination with altered DNA repair genes, makes it harder for cancer cells to survive. Enzalutamide works by blocking hormones from fueling cancer cell growth.
The approval, announced on June 20, makes talazoparib the third PARP-blocking drug to be cleared by the agency to treat prostate cancer. FDA based the approval on a set of data from a large, randomized phase 3 clinical trial called TALAPRO-2—funded by Pfizer, the maker of talazoparib.
The trial included two separate cohorts of participants, one of which enrolled only men whose tumors had alterations in DNA repair genes.
The new approval was based on findings from this cohort, in which participants treated with the drug combination lived longer without their cancer getting worse than those treated with the standard treatment of enzalutamide alone. At nearly 3 years after starting the talazoparib and enzalutamide treatment, about 50% of patients whose tumors had one of these changes were still alive without their cancer getting worse. For those who received enzalutamide alone, that number was about 20%.
Findings from this part of the trial were presented in June at the 2023 American Society of Clinical Oncology annual meeting.
About a quarter of men with prostate cancer have an alteration in a DNA repair gene, said Neeraj Agarwal, M.D., a medical oncologist at the University of Utah Huntsman Cancer Institute, who co-led the study. Because prostate cancer is so common, he added, many people will be eligible to receive the combination treatment even with approval limited to this subset of patients.
But some experts in prostate cancer treatment cautioned that patients in the trial have not been followed long enough to know if the drug combination improves how long patients live overall.
That’s an important missing piece of information, said Fatima Karzai, M.D., of the Genitourinary Malignancies Branch in NCI’s Center for Cancer Research. The data “need more time so we know how participants who may harbor these alterations will do,” Dr. Karzai explained.
In particular, it’s unclear if the enzalutamide–talazoparib combination provides more benefit to patients than the current practice of using enzalutamide as an initial treatment followed by a PARP inhibitor only after their cancer starts to get worse.
This latter option, she noted, offsets some of the combination’s side effects, which were more severe than those of the standard treatment.
Treatment intensification to prevent progression
In 2020, FDA approved the first PARP inhibitors for the treatment of metastatic castration-resistant prostate cancer with altered DNA repair genes. Both approvals covered use of the drugs as second-line treatments—that is, only for patients whose cancer was no longer responding to an earlier hormone therapy.
Although the initial approvals were promising, Dr. Agarwal said, he wondered whether waiting to offer PARP inhibitors until after the standard treatment stopped working might be preventing these therapies from helping more patients; as many as 40% of these patients stop treatments when their disease progresses. This high attrition, he noted, is one of the reasons why using stronger therapy up front has worked in advanced prostate cancer.
So, he wanted to test using a PARP inhibitor during initial therapy, when more patients are feeling better and more enthusiastic about receiving it, he said.
Rather than using a PARP inhibitor alone as an initial treatment, he continued, they decided to combine it with a powerful hormone therapy (in this case enzalutamide), a decision based on data from laboratory studies showing that these drugs may be even more effective when combined.
The researchers enrolled two different cohorts of participants to test the combination therapy in a broader cohort of men and in those who are most likely to benefit from the new treatment. The first cohort included 805 men with metastatic castration-resistant prostate cancer, regardless of whether their tumors had any alterations in 12 specific DNA repair genes. The trial’s second cohort was limited to 399 men whose tumors had alterations in any of the 12 genes.
Participants in both cohorts received either enzalutamide plus talazoparib or enzalutamide plus a placebo, given by pill once daily. Then, the researchers measured the length of time until cancer growth could be seen on standard imaging scans, called radiographic progression-free survival.
In the cohort of men whose tumors had alterations in DNA repair genes—the part of the trial on which the new approval is based—the median amount of time until cancer growth could be seen on a scan was about 14 months for those who received enzalutamide alone. But among those who received the combination treatment, too few people had experienced a worsening of their cancer to even determine the median radiographic profession-free survival in this cohort.
Importantly, on standardized questionnaires given to these patients, those who received enzalutamide and talazoparib reported a better quality of life for longer than patients treated only with enzalutamide, Dr. Agarwal noted.
Although the drug combination was approved for people whose tumors have alterations in any of 12 DNA repair genes, when the researchers looked at the specific genes that were altered, they found that men with alterations to the BRCA1, BRCA2, or CDK12 genes improved most from the combination treatment, said study co-lead Karim Fizazi, M.D., Ph.D., of the Institut Gustave Roussy in France, during his presentation of the second cohort findings at the ASCO meeting.
About half of the participants in the second cohort had an alteration in at least one of the BRCA1, BRCA2, or CDK12 genes.
Weighing the risks of PARP inhibitor side effects
The enzalutamide–talazoparib combination is another option for people with metastatic castration-resistant prostate cancer that has these DNA damage repair alterations, “which is great,” said Dr. Karzai. But, she added, it’s critical for people to consider their quality of life and let their physicians know when they don’t feel well on the combination therapy.
“I think a lot of patients are afraid, especially when treated with drug combinations, that the doctor will take them off the drugs if they say anything about side effects,” Dr. Karzai said. “But I think it's really important that these patient outcomes are reported.”
PARP inhibitors can lead to a few serious side effects, explained Dr. Agarwal. These include a drop in blood cell counts, nausea and vomiting, and fatigue. Talazoparib, he noted, tends to cause substantial drops in red and white blood cell counts.
In the cohort of men whose tumors have a DNA repair gene alteration, about 10% of patients discontinued the combination treatment due to severe side effects of any kind, compared with 7% in the placebo group. The most common side effect was anemia, which occurred in nearly two-thirds of people receiving the drug combination—four times the number of people who developed anemia on enzalutamide alone.
The onset of anemia happens soon after starting treatment, Dr. Agarwal said. Typically, the oncologist will lower the dose, he explained, and at that point most patients are able to tolerate talazoparib well. In this cohort, he noted, only 4% of patients stopped receiving talazoparib due to anemia after initial dose reductions.
A broader approval remains uncertain
For now, FDA’s approval applies only to men whose metastatic castration-resistant prostate cancer has DNA repair gene alterations. But patients whose tumors lacked alterations in DNA repair genes also had improved radiographic progression-free survival when treated with the combination compared with those treated with enzalutamide alone—although not nearly to the same extent, Dr. Agarwal noted.
Findings from this larger cohort in the trial were published June 4 in The Lancet.
So, an important next step, he said, is to understand why these tumors responded to the treatment despite lacking this critical genetic change. If they can identify specific biological characteristics of patients’ tumors that make them more likely to respond to the drug, it may be possible to expand the number of people who can benefit from the combination.
But for now, he said, “we need more data on safety and long-term overall survival benefit for all patients before we can recommend this combination [to everyone].”
The importance of genetic testing
Even as researchers try to iron out some of the unknowns about how best to use PARP inhibitors in people with metastatic castration-resistant prostate cancer, one thing is clear, Dr. Karzai emphasized.
“Everybody who's getting diagnosed with metastatic prostate cancer should talk to their doctors about getting genetic testing,” from tumor biopsies and through specialized tests that identify inherited mutations that may be present in the cells of the body and have been present since birth, she urged.
Dr. Agarwal agreed, explaining that many people being treated in smaller hospitals and cancer centers in their own communities are not getting tested for DNA repair gene alterations.
“Maybe because of a lack of resources, a lack of awareness, I don't know why, but a significant number of patients are not getting tested in the community,” he said.
Testing tumors for specific genetic changes is important for two reasons, Dr. Agarwal said. First, as more targeted drugs enter the market, it is increasingly possible to target the specific alterations that drive someone’s tumor. And second, some of these genetic alterations, known as germline alterations, are associated with familial predisposition to the disease, which may prompt screening in other family members.
“For metastatic prostate cancer, everybody agrees now that they need genomic testing of the tumor and germline,” Dr. Agarwal emphasized.
Pfizer’s TALZENNA® in Combination with XTANDI® Prolongs Overall Survival in Phase 3 TALAPRO-2 Trial
- First and only PARP inhibitor plus ARPI combination to demonstrate statistically significant overall survival (OS) benefit in patients with metastatic castration-resistant prostate cancer (mCRPC)
- Results to be shared with global health authorities to potentially update the TALZENNA label
NEW YORK--(BUSINESS WIRE)--Pfizer Inc. (NYSE: PFE) today announced positive topline results from the final prespecified overall survival (OS) analysis of the TALAPRO-2 study of TALZENNA® (talazoparib), an oral poly ADP-ribose polymerase (PARP) inhibitor, in combination with XTANDI® (enzalutamide), an androgen receptor pathway inhibitor (ARPI), in patients with metastatic castration-resistant prostate cancer (mCRPC). Results showed a statistically significant and clinically meaningful improvement in the final OS in all-comers (cohort 1) as well as in those patients with homologous recombination repair (HRR) gene-mutated mCRPC (cohort 2), compared to XTANDI alone.
“The TALAPRO-2 results showed that TALZENNA plus XTANDI is the first and only PARP inhibitor in combination with an ARPI to significantly improve survival in patients with metastatic castration-resistant prostate cancer, regardless of mutation status,” said Roger Dansey, M.D., Chief Development Officer, Oncology, Pfizer. “Pfizer is dedicated to advancing scientific breakthroughs in genitourinary cancers, and these exciting TALAPRO-2 results further highlight our long-standing commitment to improving survival for men with prostate cancer.”
“These overall survival results indicate potentially practice-changing efficacy for TALZENNA in combination with XTANDI for men with metastatic castration-resistant prostate cancer,” said Neeraj Agarwal, M.D., FASCO, Professor and Presidential Endowed Chair of Cancer Research at Huntsman Cancer Institute, University of Utah, and global lead investigator for TALAPRO-2. “Metastatic castration-resistant prostate cancer is the most advanced and aggressive stage of the disease, and the TALAPRO-2 results provide much-needed hope to patients who remain in high unmet need for effective treatment options.”
At the time of the final analysis, the clinically meaningful improvement in radiographic progression free survival (rPFS) was maintained in both cohorts from the prior primary analysis previously reported and published in The Lancet. In addition, the safety profile of TALZENNA plus XTANDI was generally consistent with the known safety profile of each medicine. Detailed results from TALAPRO-2 will be submitted for presentation at an upcoming medical congress. These data will also be shared with global health authorities to potentially support regulatory filings to update and potentially expand the approved label for TALZENNA.
TALZENNA in combination with XTANDI was approved by the U.S. Food and Drug Administration (FDA) for the treatment of adult patients with HRR gene-mutated mCRPC in June 2023. The combination was also approved by the European Commission in January 2024 for the treatment of adult patients with mCRPC in whom chemotherapy is not clinically indicated. TALZENNA is the first and only PARP inhibitor licensed in the European Union for use with XTANDI for patients with mCRPC, with or without gene mutations. TALZENNA in combination with XTANDI is now approved in more than 35 countries globally for patients with mCRPC.
About Metastatic Castration-Resistant Prostate Cancer
Prostate cancer is the second most common cancer in men and the fifth most common cause of cancer death among men worldwide, with an estimated 1.4 million new cases diagnosed in 2022.1 In the U.S., it is the most common cancer in men.2 mCRPC is a cancer that has spread beyond the prostate gland and has progressed despite medical or surgical treatment to lower testosterone. Approximately 10%–20% of prostate cancer patients develop mCRPC within 5−7 years of diagnosis.3 Between 1.2%–2.1% of all prostate cancer cases globally are mCRPC.4
About TALAPRO-2
The Phase 3 TALAPRO-2 trial is a multicenter, randomized, double-blind, placebo-controlled study that enrolled 1,035 unique patients with mCRPC (who had not received new life-prolonging systemic treatments after documentation of mCRPC) at sites in the U.S., Canada, Europe, South America, and the Asia-Pacific region. The study included two patient cohorts: all-comers (n=805, of whom 169 had HRR mutations and 636 did not) and those with HRR gene mutations (n=399, including 169 patients from Cohort 1 and 230 enrolled in Cohort 2). Patients with castrate testosterone levels were randomized to receive TALZENNA 0.5 mg/day plus XTANDI 160mg/day, or placebo plus XTANDI 160mg/day.
The primary endpoint of the trial was rPFS, defined as the time from the date of randomization to first objective evidence of radiographic progression by blinded independent review, or death, whichever occurred first, in both Cohort 1 (all-comers) and Cohort 2 (those with HRRm). Secondary endpoints included OS, objective response rate (ORR), duration of response (DOR), and prostate-specific antigen (PSA) response.
For more information on the TALAPRO-2 trial (NCT03395197), go to www.clinicaltrials.gov.
About TALZENNA® (talazoparib)
TALZENNA is an oral inhibitor of poly ADP-ribose polymerase (PARP), which plays a role in DNA damage repair. Preclinical studies have demonstrated that TALZENNA blocks PARP enzyme activity and traps PARP at the site of DNA damage, leading to decreased cancer cell growth and cancer cell death.
TALZENNA is approved in the U.S., EU, and multiple other regions for the treatment of adult patients with deleterious or suspected deleterious gBRCAm HER2-negative locally advanced or metastatic breast cancer. In the U.S., TALZENNA is approved in combination with XTANDI for the treatment of adult patients with homologous recombination repair (HRR) gene-mutated metastatic castration-resistant prostate cancer (mCRPC). In the EU, TALZENNA is approved in combination with enzalutamide for the treatment of adult patients with mCRPC in whom chemotherapy is not clinically indicated.
TALZENNA® (talazoparib) Indication in the U.S.
TALZENNA is a poly (ADP-ribose) polymerase (PARP) inhibitor indicated for:
HRR gene-mutated mCRPC:
- In combination with enzalutamide for the treatment of adult patients with homologous recombination repair (HRR) gene-mutated metastatic castration-resistant prostate cancer (mCRPC).
Breast Cancer:
- As a single agent, for the treatment of adult patients with deleterious or suspected deleterious germline BRCA-mutated (gBRCAm) HER2-negative locally advanced or metastatic breast cancer. Select patients for therapy based on an FDA-approved companion diagnostic for TALZENNA.
TALZENNA® (talazoparib) Important Safety Information
WARNINGS and PRECAUTIONS
Myelodysplastic Syndrome/Acute Myeloid Leukemia (MDS/AML), including cases with a fatal outcome, has been reported in patients who received TALZENNA. Overall, MDS/AML has been reported in 0.4% (3 out of 788) of solid tumor patients treated with TALZENNA as a single agent in clinical studies. In TALAPRO-2, MDS/AML occurred in 2 out of 511 (0.4%) patients treated with TALZENNA and enzalutamide and in 0 out of 517 (0%) patients treated with placebo and enzalutamide. The durations of TALZENNA treatment in these five patients prior to developing MDS/AML were 0.3, 1, 2, 3, and 5 years, respectively. Most of these patients had received previous chemotherapy with platinum agents and/or other DNA damaging agents including radiotherapy.
Do not start TALZENNA until patients have adequately recovered from hematological toxicity caused by previous chemotherapy. Monitor blood counts monthly during treatment with TALZENNA. For prolonged hematological toxicities, interrupt TALZENNA and monitor blood counts weekly until recovery. If counts do not recover within 4 weeks, refer the patient to a hematologist for further investigations including bone marrow analysis and blood sample for cytogenetics. If MDS/AML is confirmed, discontinue TALZENNA.
Myelosuppression consisting of anemia, neutropenia, and/or thrombocytopenia have been reported in patients treated with TALZENNA. In TALAPRO-2, Grade ≥3 anemia, neutropenia, and thrombocytopenia were reported, respectively, in 45%, 18%, and 8% of patients receiving TALZENNA and enzalutamide. Overall, 39% of patients (199/511) required a red blood cell transfusion, including 22% (111/511) who required multiple transfusions. Discontinuation due to anemia, neutropenia, and thrombocytopenia occurred, respectively, in 7%, 3%, and 0.4% of patients.
Withhold TALZENNA until patients have adequately recovered from hematological toxicity caused by previous therapy. Monitor blood counts monthly during treatment with TALZENNA. If hematological toxicities do not resolve within 28 days, discontinue TALZENNA and refer the patient to a hematologist for further investigations including bone marrow analysis and blood sample for cytogenetics.
Embryo-Fetal Toxicity TALZENNA can cause fetal harm when administered to pregnant women. Advise male patients with female partners of reproductive potential or who are pregnant to use effective contraception during treatment with TALZENNA and for 4 months after receiving the last dose.
ADVERSE REACTIONS
In TALAPRO-2, serious adverse reactions reported in >2% of patients included anemia (9%) and fracture (3%). Fatal adverse reactions occurred in 1.5% of patients, including pneumonia, COVID infection, and sepsis (1 patient each).
The most common adverse reactions (≥ 10%, all Grades), including laboratory abnormalities, for patients in the TALAPRO-2 study who received TALZENNA in combination with enzalutamide vs patients receiving placebo with enzalutamide were hemoglobin decreased (79% vs 34%), neutrophils decreased (60% vs 18%), lymphocytes decreased (58% vs 36%), fatigue (49% vs 40%), platelets decreased (45% vs 8%), calcium decreased (25% vs 11%), nausea (21% vs 17%), decreased appetite (20% vs 14%), sodium decreased (22% vs 20%), phosphate decreased (17% vs 13%), fractures (14% vs 10%), magnesium decreased (14% vs 12%), dizziness (13% vs 9%), bilirubin increased (11% vs 7%), potassium decreased (11% vs 7%), and dysgeusia (10% vs 4.5%).
Clinically relevant adverse reactions in <10% of patients who received TALZENNA with enzalutamide included abdominal pain (9%), vomiting (9%), alopecia (7%), dyspepsia (4%), venous thromboembolism (3%) and stomatitis (2%).
Based on animal studies, TALZENNA may impair fertility in males of reproductive potential.
DRUG INTERACTIONS
Coadministration with P-gp inhibitors The effect of coadministration of P-gp inhibitors on talazoparib exposure when TALZENNA is taken in combination with enzalutamide has not been studied. Monitor patients for increased adverse reactions and modify the dosage as recommended for adverse reactions when TALZENNA is coadministered with a P-gp inhibitor.
Coadministration with BCRP inhibitors Monitor patients for increased adverse reactions and modify the dosage as recommended for adverse reactions when TALZENNA is coadministered with a BCRP inhibitor. Coadministration of TALZENNA with BCRP inhibitors may increase talazoparib exposure, which may increase the risk of adverse reactions.
USE IN SPECIFIC POPULATIONS
Renal Impairment The recommended dosage of TALZENNA for patients with moderate renal impairment (CLcr 30 - 59 mL/min) is 0.35 mg taken orally once daily in combination with enzalutamide. The recommended dosage of TALZENNA for patients with severe renal impairment (CLcr 15 - 29 mL/min) is 0.25 mg taken orally once daily in combination with enzalutamide. No dose adjustment is required for patients with mild renal impairment. TALZENNA has not been studied in patients requiring hemodialysis.
Please see full U.S. Prescribing Information and Patient Information for TALZENNA® (talazoparib) at www.TALZENNA.com.
About XTANDI® (enzalutamide) and Important Safety Information
XTANDI® (enzalutamide) is an androgen receptor signaling inhibitor. XTANDI is a standard of care and has received regulatory approvals in one or more countries around the world for use in men with metastatic castration-sensitive prostate cancer (mCSPC; also known as metastatic hormone-sensitive prostate cancer or mHSPC), metastatic castration-resistant prostate cancer (mCRPC), non-metastatic castration-resistant prostate cancer (nmCRPC) and nonmetastatic castration-sensitive prostate cancer (nmCSPC) with biochemical recurrence at high risk for metastasis (high-risk BCR). XTANDI is currently approved for one or more of these indications in more than 90 countries, including in the U.S., EU, and Japan. Over one million patients have been treated with XTANDI globally.5
Warnings and Precautions
Seizure occurred in 0.6% of patients receiving XTANDI in eight randomized clinical trials. In a study of patients with predisposing factors for seizure, 2.2% of XTANDI-treated patients experienced a seizure. It is unknown whether anti-epileptic medications will prevent seizures with XTANDI. Patients in the study had one or more of the following predisposing factors: use of medications that may lower the seizure threshold, history of traumatic brain or head injury, history of cerebrovascular accident or transient ischemic attack, and Alzheimer’s disease, meningioma, or leptomeningeal disease from prostate cancer, unexplained loss of consciousness within the last 12 months, history of seizure, presence of a space occupying lesion of the brain, history of arteriovenous malformation, or history of brain infection. Advise patients of the risk of developing a seizure while taking XTANDI and of engaging in any activity where sudden loss of consciousness could cause serious harm to themselves or others. Permanently discontinue XTANDI in patients who develop a seizure during treatment.
Posterior Reversible Encephalopathy Syndrome (PRES) There have been reports of PRES in patients receiving XTANDI. PRES is a neurological disorder that can present with rapidly evolving symptoms including seizure, headache, lethargy, confusion, blindness, and other visual and neurological disturbances, with or without associated hypertension. A diagnosis of PRES requires confirmation by brain imaging, preferably MRI. Discontinue XTANDI in patients who develop PRES.
Hypersensitivity reactions, including edema of the face (0.5%), tongue (0.1%), or lip (0.1%) have been observed with XTANDI in eight randomized clinical trials. Pharyngeal edema has been reported in post-marketing cases. Advise patients who experience any symptoms of hypersensitivity to temporarily discontinue XTANDI and promptly seek medical care. Permanently discontinue XTANDI for serious hypersensitivity reactions.
Ischemic Heart Disease In the combined data of five randomized, placebo-controlled clinical studies, ischemic heart disease occurred more commonly in patients on the XTANDI arm compared to patients on the placebo arm (3.5% vs 2%). Grade 3-4 ischemic events occurred in 1.8% of patients on XTANDI versus 1.1% on placebo. Ischemic events led to death in 0.4% of patients on XTANDI compared to 0.1% on placebo. Monitor for signs and symptoms of ischemic heart disease. Optimize management of cardiovascular risk factors, such as hypertension, diabetes, or dyslipidemia. Discontinue XTANDI for Grade 3-4 ischemic heart disease.
Falls and Fractures occurred in patients receiving XTANDI. Evaluate patients for fracture and fall risk. Monitor and manage patients at risk for fractures according to established treatment guidelines and consider use of bone-targeted agents. In the combined data of five randomized, placebo-controlled clinical studies, falls occurred in 12% of patients treated with XTANDI compared to 6% of patients treated with placebo. Fractures occurred in 13% of patients treated with XTANDI and in 6% of patients treated with placebo.
Embryo-Fetal Toxicity The safety and efficacy of XTANDI have not been established in females. XTANDI can cause fetal harm and loss of pregnancy when administered to a pregnant female. Advise males with female partners of reproductive potential to use effective contraception during treatment with XTANDI and for 3 months after the last dose of XTANDI.
Adverse Reactions (ARs) the data from the five randomized placebo-controlled trials, the most common ARs (≥ 10%) that occurred more frequently (≥ 2% over placebo) in XTANDI-treated patients were musculoskeletal pain, fatigue, hot flush, constipation, decreased appetite, diarrhea, hypertension, hemorrhage, fall, fracture, and headache. In the bicalutamide-controlled study, the most common ARs (≥ 10%) reported in XTANDI-treated patients were asthenia/fatigue, back pain, musculoskeletal pain, hot flush, hypertension, nausea, constipation, diarrhea, upper respiratory tract infection, and weight loss.
In AFFIRM, the placebo-controlled study of metastatic CRPC (mCRPC) patients who previously received docetaxel, Grade 3 and higher ARs were reported among 47% of XTANDI-treated patients. Discontinuations due to ARs were reported for 16% of XTANDI-treated patients. In PREVAIL, the placebo-controlled study of chemotherapy-naive mCRPC patients, Grade 3-4 ARs were reported in 44% of XTANDI patients and 37% of placebo patients. Discontinuations due to ARs were reported for 6% of XTANDI-treated patients. In TERRAIN, the bicalutamide-controlled study of chemotherapy-naive mCRPC patients, Grade 3-4 ARs were reported in 39% of XTANDI patients and 38% of bicalutamide patients. Discontinuations with an AR as the primary reason were reported for 8% of XTANDI patients and 6% of bicalutamide patients.
In PROSPER, the placebo-controlled study of nonmetastatic CRPC (nmCRPC) patients, Grade 3 or higher ARs were reported in 31% of XTANDI patients and 23% of placebo patients. Discontinuations with an AR as the primary reason were reported for 9% of XTANDI patients and 6% of placebo patients.
In ARCHES, the placebo-controlled study of metastatic CSPC (mCSPC) patients, Grade 3 or higher ARs were reported in 24% of XTANDI-treated patients. Permanent discontinuation due to ARs as the primary reason was reported in 5% of XTANDI patients and 4% of placebo patients.
In EMBARK, the placebo-controlled study of nonmetastatic CSPC (nmCSPC) with high-risk biochemical recurrence (BCR) patients, Grade 3 or higher adverse reactions during the total duration of treatment were reported in 46% of patients treated with XTANDI plus leuprolide, 50% of patients receiving XTANDI as a single agent, and 43% of patients receiving placebo plus leuprolide. Permanent treatment discontinuation due to adverse reactions during the total duration of treatment as the primary reason was reported in 21% of patients treated with XTANDI plus leuprolide, 18% of patients receiving XTANDI as a single agent, and 10% of patients receiving placebo plus leuprolide.
Lab Abnormalities: Lab abnormalities that occurred in ≥ 5% of patients, and more frequently (> 2%) in the XTANDI arm compared to placebo in the pooled, randomized, placebo-controlled studies are hemoglobin decrease, neutrophil count decreased, white blood cell decreased, hyperglycemia, hypermagnesemia, hyponatremia, hyperphosphatemia, and hypercalcemia.
Hypertension: In the combined data from five randomized placebo-controlled clinical trials, hypertension was reported in 14.2% of XTANDI patients and 7.4% of placebo patients. Hypertension led to study discontinuation in < 1% of patients in each arm.
Drug Interactions
Effect of Other Drugs on XTANDI Avoid coadministration with strong CYP2C8 inhibitors. If coadministration cannot be avoided, reduce the dosage of XTANDI.
Avoid coadministration with strong CYP3A4 inducers. If coadministration cannot be avoided, increase the dosage of XTANDI.
Effect of XTANDI on Other Drugs Avoid coadministration with certain CYP3A4, CYP2C9, and CYP2C19 substrates for which minimal decrease in concentration may lead to therapeutic failure of the substrate. If coadministration cannot be avoided, increase the dosage of these substrates in accordance with their Prescribing Information. In cases where active metabolites are formed, there may be increased exposure to the active metabolites.
Please access this link for XTANDI’S US Full Prescribing Information for additional safety information.
About Pfizer Oncology
At Pfizer Oncology, we are at the forefront of a new era in cancer care. Our industry-leading portfolio and extensive pipeline includes three core mechanisms of action to attack cancer from multiple angles, including small molecules, antibody-drug conjugates (ADCs), and bispecific antibodies, including other immune-oncology biologics. We are focused on delivering transformative therapies in some of the world’s most common cancers, including breast cancer, genitourinary cancer, hematology-oncology, and thoracic cancers, which includes lung cancer. Driven by science, we are committed to accelerating breakthroughs to help people with cancer live better and longer lives.
About Pfizer: Breakthroughs That Change Patients’ Lives
At Pfizer, we apply science and our global resources to bring therapies to people that extend and significantly improve their lives. We strive to set the standard for quality, safety and value in the discovery, development, and manufacture of health care products, including innovative medicines and vaccines. Every day, Pfizer colleagues work across developed and emerging markets to advance wellness, prevention, treatments, and cures that challenge the most feared diseases of our time. Consistent with our responsibility as one of the world’s premier innovative biopharmaceutical companies, we collaborate with health care providers, governments, and local communities to support and expand access to reliable, affordable health care around the world. For 175 years, we have worked to make a difference for all who rely on us. We routinely post information that may be important to investors on our website at www.Pfizer.com. In addition, to learn more, please visit us on www.Pfizer.com and follow us on X at @Pfizer and @Pfizer News, LinkedIn, YouTube and like us on Facebook at Facebook.com/Pfizer.
About the Pfizer/Astellas Collaboration
In October 2009, Medivation, Inc., which is now part of Pfizer (NYSE: PFE), and Astellas (TSE: 4503) entered into a global agreement to jointly develop and commercialize XTANDI® (enzalutamide). The companies jointly commercialize XTANDI in the United States, and Astellas has responsibility for manufacturing and all additional regulatory filings globally, as well as commercializing XTANDI outside the United States.
Disclosure Notice
The information contained in this release is as of October 10, 2024. Pfizer assumes no obligation to update forward-looking statements contained in this release as the result of new information or future events or developments.
This release contains forward-looking information about Pfizer Oncology, TALZENNA and XTANDI, including their potential benefits, the TALAPRO-2 results and plans to share the results with global health authorities to potentially update the TALZENNA label, that involves substantial risks and uncertainties that could cause actual results to differ materially from those expressed or implied by such statements.
Contacts
Media Contact:
+1 (212) 733-1226
PfizerMediaRelations@Pfizer.com
Investor Contact:
+1 (212) 733-4848
IR@Pfizer.com
TALZENNA- talazoparib capsule
Pfizer Laboratories Div Pfizer Inc
----------
| This Patient Information has been approved by the U.S. Food and Drug Administration Revised: 2/2024 | |
PATIENT INFORMATION | |
What is the most important information I should know about TALZENNA? Bone marrow problems called Myelodysplastic Syndrome (MDS) or Acute Myeloid Leukemia (AML).
Some people who have cancer and who have received previous treatment
with chemotherapy or certain other medicines for their cancer have
developed MDS or AML during or after treatment with TALZENNA. MDS or AML
may lead to death. If you develop MDS or AML, your healthcare provider
will stop treatment with TALZENNA. | |
|
|
Your healthcare provider will do blood tests to check your blood cell counts:
See "What are the possible side effects of TALZENNA?" below for other side effects of TALZENNA. | |
What is TALZENNA?
Your healthcare provider will perform a test to make sure that TALZENNA is right for you. It is not known if TALZENNA is safe and effective in children. | |
Before taking TALZENNA, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take,
including prescription medicines, over-the-counter medicines, vitamins,
and herbal supplements. Taking TALZENNA and certain other medicines can
affect how TALZENNA works and may cause side effects. | |
How should I take TALZENNA?
| |
What are the possible side effects of TALZENNA? TALZENNA may cause serious side effects, including: The most common side effects of TALZENNA when taken alone include: | |
|
|
The most common side effects of TALZENNA when taken in combination with enzalutamide include: | |
|
|
TALZENNA may cause fertility problems in males. This may affect your ability to father a child. Talk to your healthcare provider if this is a concern for you. These are not all of the possible side effects of TALZENNA. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. | |
How should I store TALZENNA?
Keep TALZENNA and all medicines out of the reach of children. | |
General information about the safe and effective use of TALZENNA. | |
What are the ingredients in TALZENNA?
| |




Comments
Post a Comment