FDA Expands Pluvicto Approval: New Hope for mCRPC Patients Before Chemotherapy
FDA Expands Pluvicto Approval: New Hope for mCRPC Patients Before Chemotherapy
March 28, 2025
In a significant advancement for prostate cancer treatment, the U.S. Food and Drug Administration (FDA) has expanded the approval of Pluvicto (lutetium Lu 177 vipivotide tetraxetan) to include patients with prostate-specific membrane antigen (PSMA)–positive metastatic castration-resistant prostate cancer (mCRPC) who have received prior androgen receptor pathway inhibitor (ARPI) therapy and are considered appropriate to delay taxane-based chemotherapy.
What This Means for Patients
This expanded approval represents a major shift in treatment options, allowing eligible patients to access this targeted radioligand therapy earlier in their treatment journey, potentially delaying the need for chemotherapy with its associated side effects.
The approval is based on results from the Phase III PSMAfore trial, which showed that Pluvicto significantly improved radiographic progression-free survival (rPFS) compared to changing to a different ARPI. Patients treated with Pluvicto experienced a median rPFS of 9.3 months compared to 5.6 months for those who switched to a different ARPI therapy.
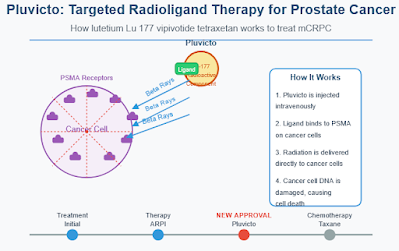
How Pluvicto Works
Pluvicto is a precision medicine approach known as a radioligand therapy. It combines two components:
- A targeting molecule that binds specifically to PSMA, a protein found in high amounts on prostate cancer cells
- A radioactive particle (lutetium-177) that delivers radiation directly to the cancer cells
This targeted approach allows the treatment to deliver radiation precisely to cancer cells throughout the body while limiting damage to surrounding healthy tissue.
Patient Selection
Patients must undergo a PSMA PET scan to determine if they have PSMA-positive tumors before receiving Pluvicto. This diagnostic test uses gallium Ga 68 gozetotide (Locametz) or another approved PSMA PET product to identify appropriate candidates for treatment.
The recommended dosage is 7.4 GBq (200 mCi) administered intravenously every 6 weeks for up to 6 doses.
Side Effects
The most common side effects reported with Pluvicto include dry mouth, nausea, fatigue, and anemia. Patients should be monitored for potential renal toxicity and decreased blood cell counts during treatment.
Looking Forward
This expanded approval represents the continuing evolution of precision medicine approaches for prostate cancer. Researchers are currently conducting additional clinical trials to evaluate Pluvicto in even earlier stages of prostate cancer treatment, including in hormone-sensitive metastatic disease and oligometastatic settings.
The IPCSG will be hosting an informational session on this new treatment option at our next monthly meeting. A medical oncologist specializing in advanced prostate cancer will be available to answer questions about this treatment.
Sources:
-
FDA expands Pluvicto's metastatic castration-resistant prostate cancer indication. FDA. March 28, 2025. https://tinyurl.com/y77tmenc
-
FDA approves Novartis radioligand therapy Pluvicto for earlier use before chemotherapy in PSMA-positive metastatic castration-resistant prostate cancer. Novartis. March 28, 2025. https://www.novartis.com/news/media-releases/fda-approves-novartis-radioligand-therapy-pluvicto-earlier-use-chemotherapy-psma-positive-metastatic-castration-resistant-prostate-cancer
-
Novartis Pluvicto™ shows clinically meaningful and highly statistically significant rPFS benefit in patients with PSMA-positive metastatic castration-resistant prostate cancer in the pre-taxane setting. Novartis. October 23, 2023. https://www.novartis.com/news/media-releases/novartis-pluvictotm-shows-clinically-meaningful-and-highly-statistically-significant-rpfs-benefit-patients-psma-positive-metastatic-castration-resistant-prostate-cancer-pre-taxane-setting
-
Lutetium-177 (Lu-177) PSMA therapy for Prostate Cancer (Pluvicto). UChicago Medicine. https://www.uchicagomedicine.org/cancer/types-treatments/prostate-cancer/treatment/lutetium-177-psma-therapy-for-prostate-cancer

Comments
Post a Comment