Major Breakthrough: AI-Powered Urine Test Shows 92% Accuracy in Prostate Cancer Detection
Revolutionary Research Could Transform Early Diagnosis and Reduce Unnecessary Biopsies
August 2025 — A groundbreaking international study published in Cancer Research represents a potential paradigm shift in prostate cancer diagnosis, offering hope for earlier detection through a simple, non-invasive urine test that significantly outperforms current PSA blood testing.
The Breakthrough
Researchers from Karolinska Institutet (Sweden), Imperial College London (UK), and Xiyuan Hospital at the China Academy of Chinese Medical Sciences have identified a set of highly accurate biomarkers for prostate cancer that achieved an AUC of 0.92 for prostate cancer detection in urine samples. This represents a significant improvement over traditional PSA testing, which has long struggled with both false positives and missed diagnoses.
The study, led by Dr. Mikael Benson, senior researcher at the Department of Clinical Science, Intervention and Technology at Karolinska Institutet, analyzed data from nearly 2,000 patients using advanced techniques including spatial transcriptomics, pseudotime modeling, and artificial intelligence.
How It Works
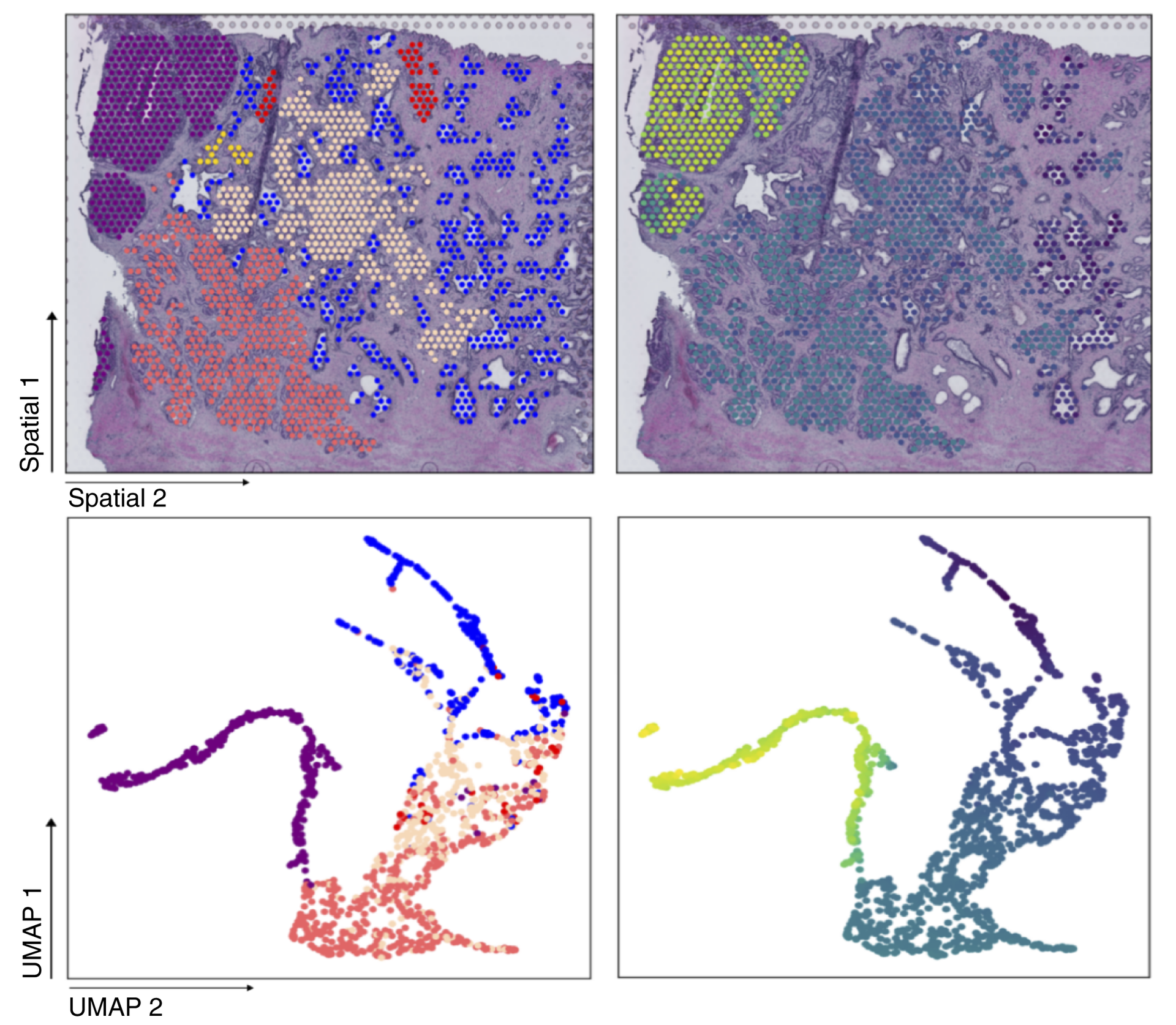
The researchers constructed pseudotime models based on spatial transcriptomic data from three independent prostate cancer studies to prioritize genes most correlated with malignant transformation. The identified genes were associated with cancer grade, copy number aberrations, hallmark pathways, and drug targets.
Using spatial transcriptomics (ST), the investigators developed computational timelines of malignant transformation called pseudotime (PT) models and then applied machine learning to identify 45 genes associated with cancer grade, chromosomal changes, and pathways relevant to prostate cancer.
Key biomarkers identified include SPON2, AMACR, and TMEFF2, which showed consistent patterns of overexpression across independent patient cohorts. SPON2, for example, showed higher expression in high-grade cancer tissue compared to healthy or benign samples.
Clinical Advantages
Dr. Benson emphasized the practical benefits: "There are many advantages to measuring biomarkers in urine. It's non-invasive and painless and can potentially be done at home. The sample can then be analysed using routine methods in clinical labs".
The research offers several key advantages over current diagnostic methods:
- Higher accuracy: Machine learning-based prediction models revealed that the biomarkers in urine had an AUC of 0.92 for prostate cancer
- Non-invasive testing: Eliminates the need for painful biopsies in many cases
- At-home collection potential: Could enable convenient screening
- Grade assessment: The biomarkers were associated with cancer grade, helping determine disease severity
Parallel Industry Developments
The research comes alongside other promising developments in AI-powered prostate cancer diagnostics. PanGIA Biotech presented findings at the 2025 Annual Meeting of the American Society of Clinical Oncology (ASCO) from a prospective, multi-center validation study evaluating its AI-powered, urine-based platform. The study achieved an F1 score of 0.843 with a recall of 0.967 in distinguishing cancer from non-cancer subjects in 197 biopsy-confirmed prostate cancer patients and 84 healthy controls.
Additionally, earlier work from the Korea Institute of Science and Technology (KIST) developed an AI smart biosensor that can detect prostate cancer using urine tests with 95.5 percent accuracy in 76 urine samples.
Integration with Major Clinical Trials
The timing of this breakthrough aligns perfectly with the launch of the TRANSFORM trial, a £42 million research programme by Prostate Cancer UK to find the best way to screen men for prostate cancer. It will be the biggest trial in prostate cancer screening for 20 years.
Large-scale clinical trials are being planned for the next phase of the research. One such is being discussed with Professor Rakesh Heer of Imperial College London, co-author of the study and head of TRANSFORM, the UK's national prostate cancer study, which offers a platform for expediting the testing of promising biomarkers.
The TRANSFORM trial will compare multiple screening options to each other and the current system, to find the safest, most accurate and most cost-effective way to screen men for prostate cancer. The massive scale of the trial will also enable the collection of a hugely important 'bio bank' of data and images to help drive new discoveries.
Addressing Health Disparities
Both the research and TRANSFORM trial specifically address health disparities. One in 4 black men will develop prostate cancer - double the risk of other men. Therefore, to ensure the trial helps reduce their risk of dying from this disease, 1 in 10 men invited to participate will be black men. Participating men in the screening trial will be aged 50 to 75, with black men eligible from the lower age range of 45 to 75.
Impact on Clinical Practice
Dr. Benson stated: "New, more precise biomarkers than PSA can lead to earlier diagnosis and better prognoses for men with prostate cancer. Moreover, it can reduce the number of unnecessary prostate biopsies in healthy men".
This development addresses a critical need in prostate cancer care, where PSA testing is associated with decreased mortality, but its lack of sensitivity and specificity has resulted in many unnecessary biopsies or missed prostate cancer diagnoses.
Future Therapeutic Applications
The new tool could also help enhance the delivery of more personalized treatment in the future because some of the biomarkers are linked to known drug targets. "This raises the possibility that ST and PT can be used to identify drug targets and that the protein levels of these targets may be used as a companion diagnostic tool to personalize treatment with such drugs," the researchers wrote.
Next Steps and Timeline
The study should be further tested in prospective studies, with researchers making all data and analytical tools publicly available and preparing for large-scale clinical trials.
The TRANSFORM trial is due to start in spring 2024 with recruitment likely to begin in autumn 2024, offering a potential pathway for validating these new biomarkers at scale.
Timeline to Clinical Practice in the United States
While the breakthrough research shows tremendous promise, patients should understand the realistic timeline for availability in U.S. clinical practice. The pathway from laboratory discovery to FDA-approved diagnostic test involves multiple regulatory and validation steps that typically span 5-10 years.
Current Regulatory Status: The Karolinska Institute biomarkers are currently in the research phase and have not yet entered formal clinical trials required for FDA approval. The study published in Cancer Research represents pre-clinical validation across nearly 2,000 patients, which is substantial but not sufficient for regulatory approval.
Required Steps for FDA Approval:
Phase I Clinical Validation (1-2 years): The biomarkers must undergo formal clinical trials testing the diagnostic performance in a prospective study design. This phase focuses on analytical validation - ensuring the test performs consistently and accurately across different laboratories and patient populations.
Phase II/III Clinical Studies (2-4 years): Large-scale prospective trials must demonstrate clinical utility - that using the biomarkers actually improves patient outcomes compared to current standard of care. The planned integration with the TRANSFORM trial could potentially accelerate this phase.
FDA Regulatory Review (6 months - 2 years): The diagnostic test must receive either 510(k) clearance or Premarket Approval (PMA) from FDA. Given the novel nature of these biomarkers, PMA approval (the more rigorous pathway) would likely be required, potentially taking 1-2 years.
Current U.S. Landscape: The FDA has already approved several prostate cancer biomarker tests, providing a regulatory precedent:
- PCA3 urine test (2012) for repeat biopsy decisions
- Prostate Health Index (PHI) for blood-based screening
- Various tissue-based genomic tests (Oncotype DX, Prolaris, Decipher)
Potential Accelerated Pathways: The FDA offers several expedited approval pathways for breakthrough diagnostics:
- Breakthrough Device Designation: Could reduce review time if the test demonstrates significant advantages over existing methods
- De Novo Classification: For novel diagnostic categories not previously regulated
Laboratory-Developed Test (LDT) Option: In the interim, the test could potentially become available through CLIA-certified laboratories as an LDT, similar to how many current genomic tests entered the market. However, FDA has indicated increased oversight of LDTs, particularly for high-complexity tests.
Industry Parallels: The PanGIA Biotech urine test, which showed similar AI-driven results at ASCO 2025, demonstrates that multiple companies are pursuing FDA approval for advanced urine-based prostate cancer diagnostics. This competitive landscape may accelerate overall development timelines.
Realistic Timeline: Based on current regulatory pathways and assuming successful clinical validation, the earliest these biomarkers could become available in U.S. clinical practice would be 3-5 years, with 5-7 years being more likely for full FDA approval and widespread adoption.
What Patients Can Do Now: While waiting for these advanced diagnostics, patients should:
- Discuss currently available biomarker tests (PHI, PCA3, 4Kscore) with their physicians
- Consider participation in clinical trials like TRANSFORM if eligible
- Stay informed about emerging diagnostic options through patient advocacy groups
Dr. Benson noted that making all data and analytical tools publicly available could help accelerate the validation process by enabling other research groups to conduct confirmatory studies in parallel.
Impact on Active Surveillance and Post-Treatment Monitoring
This breakthrough has particularly significant implications for two key patient populations:
Active Surveillance Patients: Men on active surveillance protocols currently rely on regular PSA tests, periodic biopsies, and MRI scans to monitor disease progression. The new urine-based biomarkers could revolutionize this approach by:
- Providing more frequent, non-invasive monitoring without the risks and discomfort of repeated biopsies
- Offering superior accuracy in detecting progression from low-grade to more aggressive disease
- Enabling at-home sample collection for routine surveillance, reducing clinical visits
- Potentially identifying molecular changes before they become detectable through conventional imaging or PSA changes
Since the biomarkers were associated with cancer grade and could indicate disease severity, they may provide earlier warning signals when active surveillance patients need to transition to active treatment.
Post-Treatment Recurrence Monitoring: For patients who have undergone radical prostatectomy or radiation therapy, monitoring for biochemical recurrence is critical. The new urine test offers several advantages over current PSA-based monitoring:
- Enhanced sensitivity for detecting early recurrence, particularly in cases where PSA remains undetectable but cancer cells may still be present
- Better specificity to distinguish true recurrence from benign PSA fluctuations
- Potential to detect local versus systemic recurrence patterns through biomarker profiles
- More convenient monitoring schedule with at-home urine collection
Dr. Benson noted that the biomarkers' association with specific molecular pathways and drug targets could also help guide treatment selection in recurrent disease, supporting precision medicine approaches for salvage therapy.
What This Means for Patients
For prostate cancer patients and those at risk, this research represents a significant step toward:
- Earlier, more accurate diagnosis
- Reduced need for invasive biopsies
- Better risk stratification for active surveillance decisions
- Enhanced monitoring for disease progression and recurrence
- Potential for at-home screening and surveillance
- More personalized treatment approaches based on molecular biomarkers
The combination of this breakthrough research with major clinical trials like TRANSFORM suggests that the landscape of prostate cancer diagnosis may be transformed within the next decade, offering hope for the thousands of men affected by this disease annually.
Sources and Citations
- Smelik, M., Diaz-Roncero Gonzalez, D., An, X., Heer, R., Henningsohn, L., Li, X., Wang, H., Zhao, Y., & Benson, M. (2025). Combining Spatial Transcriptomics, Pseudotime, and Machine Learning Enables Discovery of Biomarkers for Prostate Cancer. Cancer Research. DOI: 10.1158/0008-5472.CAN-25-0269. Available at: https://aacrjournals.org/cancerres/article/doi/10.1158/0008-5472.CAN-25-0269/762073/
- Karolinska Institutet. (2025, April 28). Urine test could reveal prostate cancer. ScienceDaily. https://www.sciencedaily.com/releases/2025/04/250428220907.htm
- PanGIA Biotech. (2025, June 2). PanGIA Biotech Unveils AI-Driven Urine Test Data for Early Prostate Cancer Detection at ASCO 2025. Business Wire. https://www.businesswire.com/news/home/20250602724467/en/
- Prostate Cancer UK. TRANSFORM trial. https://prostatecanceruk.org/research/transform-trial
- UK Government. (2023, November 19). Biggest prostate cancer screening trial in decades to start in UK. https://www.gov.uk/government/news/biggest-prostate-cancer-screening-trial-in-decades-to-start-in-uk
- Lee, K. H., et al. (2020). Noninvasive Precision Screening of Prostate Cancer by Urinary Multimarker Sensor and Artificial Intelligence Analysis. ACS Nano. https://pubmed.ncbi.nlm.nih.gov/33296173/
- Korea Institute of Science and Technology. (2020, December 24). KIST's AI improves prostate cancer diagnosis accuracy based on urine test. Korea Biomedical Review. https://www.koreabiomed.com/news/articleView.html?idxno=10004
- Imperial College London. New prostate cancer screening trial could save thousands of lives. https://www.imperial.ac.uk/news/253035/new-prostate-cancer-screening-trial-could/
- Inside Precision Medicine. (2025, April 30). Urine Test Detects Prostate Cancer with Greater Precision than PSA. https://www.insideprecisionmedicine.com/topics/oncology/urine-test-detects-prostate-cancer-with-greater-precision-than-psa/
- The Brighter Side of News. (2025, August 7). New urine test uses AI to diagnosis prostate cancer with 92% accuracy. https://www.thebrighterside.news/post/new-urine-test-uses-ai-to-diagnosis-prostate-cancer-with-92-accuracy/
- New urine test uses AI to diagnosis prostate cancer with 92% accuracy - The Brighter Side of News


Comments
Post a Comment