Breakthrough Nerve-Protecting Device Shows Promise for Preserving Sexual Function After Prostate Surgery
Australian surgeons pioneer use of Remplir™ collagen wrap during robotic prostatectomy procedures
For men facing prostate cancer surgery, the fear of permanent erectile dysfunction and urinary incontinence often rivals concerns about the cancer itself. Now, a biological nerve repair device originally developed for reconstructive surgery is showing promising early results in protecting the delicate nerves responsible for sexual function and bladder control during radical prostatectomy.
Remplir™, a collagen-based nerve wrap manufactured by Australian regenerative medicine company Orthocell, has been used in approximately 40 nerve-sparing robotic-assisted radical prostatectomies (RARP) across Australia. The biological device provides compression-free protection to nerves during surgery and dissolves naturally over 3 to 6 months as the tissue heals.
The Challenge: Post-Surgery Complications Remain Common
Despite advances in surgical techniques, including robotic-assisted procedures designed to spare nerves, the statistics remain sobering. Up to 80% of men experience erectile dysfunction and up to 35% suffer from urinary incontinence after radical prostatectomy due to damage of the peripheral nerves in the neurovascular bundle (NVB) surrounding the prostate.
These complications stem from injury to the neurovascular bundle, a critical cluster of nerves and blood vessels responsible for continence and sexual function, which is difficult to avoid even with nerve-sparing surgical techniques.
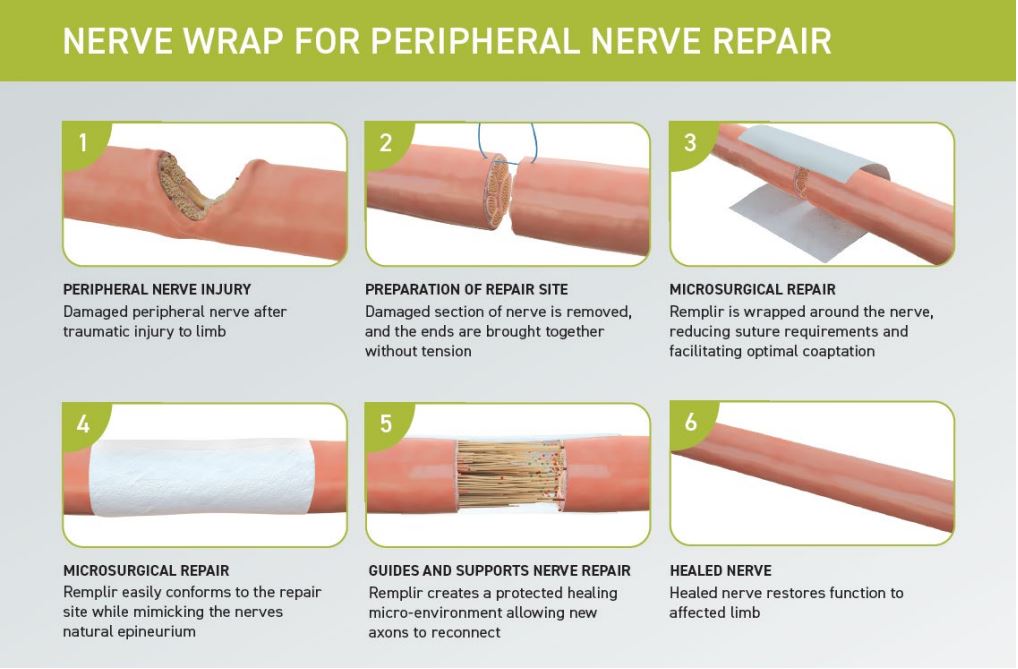
How Remplir™ Works
Remplir™ is a collagen nerve wrap manufactured using a proprietary process that preserves the collagen structure for optimal tissue integration, providing compression-free protection to the nerve and generating an ideal microenvironment to aid nerve healing.
During prostate surgery, surgeons apply the biological wrap around the neurovascular bundles to:
- Shield the nerves from surgical trauma
- Reduce post-operative inflammation
- Support nerve regeneration and healing
- Facilitate tensionless repair
Similar to peripheral nerve repair procedures that restore function to the arms and legs, Remplir can be used to protect the NVB from damage and promote restoration of normal nerve function.
Current Status and Availability
Australian Experience
Orthocell is currently collaborating with urologists to collect and analyse retrospective outcome data from the nerve-sparing procedures using Remplir in Australia, with clinical data to be released once available. The company plans to invest in further clinical studies and medical education initiatives to build evidence for this application.
Orthocell's distribution partner Device Technologies, which also supplies the da Vinci Surgical System used in most RARP procedures, provides a strategic pathway to introduce Remplir™ into urology.
U.S. Regulatory Progress
Orthocell received FDA 510(k) clearance for Remplir in April 2025, opening access to the US$1.6 billion American nerve repair market. Following FDA clearance, the company's specialist distributor network now spans 25 states, covering about 40% of the US population, with more than 40 surgeries completed across multiple hospitals and over 100 surgeons introduced to the product.
The company expects initial US revenue to begin ramping in the December quarter of 2025. However, the specific application for prostate surgery nerve protection would likely require additional clinical evidence before becoming widely adopted in the United States.
Global Expansion
Remplir is currently approved for sale in Australia, New Zealand, and Singapore, with regulatory submissions pending in Canada and Thailand. Regulatory applications for the EU and UK are on track to be submitted in the next six to 12 months.
Market Significance
Prostate cancer remains the most-diagnosed cancer among men in Australia, with more than 26,000 new cases in 2024 alone. With around 12,000 radical prostatectomies performed annually in Australia, the potential addressable market is considerable.
Orthocell is targeting a large global addressable nerve repair market estimated to be worth in excess of US$3.5 billion with around two million peripheral nerve repairs performed across major markets.
What This Means for Patients
While clinical outcome data is still being collected, the early adoption by Australian urologists suggests potential benefits for men undergoing nerve-sparing prostate surgery. The device represents a new approach to protecting nerve function during surgery rather than attempting to treat complications after they occur.
Paul Anderson, Orthocell's CEO, stated that surgeons across multiple specialties—including orthopaedics, plastic and reconstructive surgery, and now urology—are increasingly adopting Remplir to simplify procedures, minimize scarring, and improve functional recovery.
Looking Ahead
The use of Remplir™ in prostate surgery is still in its early stages, with formal clinical data pending. Patients interested in this option should discuss it with their urological surgeons, particularly those performing robotic-assisted procedures at centers participating in the ongoing data collection.
As more clinical evidence emerges and regulatory approvals expand globally, this biological nerve protection technology could potentially become a standard component of nerve-sparing prostatectomy procedures, offering hope for better preservation of quality of life after prostate cancer surgery.
Sources and References
- Orthocell Limited Press Release (September 16, 2025). "Globally Significant Opportunity Emerges as Remplir™ is used in Nerve-Sparing Prostate Cancer Surgery." PRNewswire. Available at: https://www.prnewswire.com/news-releases/globally-significant-opportunity-emerges-as-remplir-is-used-in-nerve-sparing-prostate-cancer-surgery-302557108.html
- Proactive Investors (September 17, 2025). "Orthocell's Remplir™ used in robotic prostate surgery in Australia." Available at: https://www.proactiveinvestors.com/companies/news/1078581/orthocells-remplir-used-in-robotic-prostate-surgery-in-australia.html
- Proactive Investors (September 16, 2025). "Orthocell's Remplir™ shows promise in prostate cancer surgery to reduce side effects." Available at: https://www.proactiveinvestors.com/companies/news/1078490/orthocell-s-remplir-shows-promise-in-prostate-cancer-surgery-to-reduce-side-effects-1078490.html
- Small Caps (September 16, 2025). "Orthocell's Remplir Used by Australian Urologists in Prostate Cancer Surgery." Available at: https://smallcaps.com.au/orthocell-remplir-australian-urologists-prostate-cancer-surgery/
- Orthocell Limited (April 4, 2025). "US FDA grants 510(k) clearance for Orthocell's flagship Remplir™ nerve repair product." Available at: https://orthocell.com/americas/us-fda-grants-510k-clearance-for-orthocells-flagship-remplir-nerve-repair-product/
- Proactive Investors (October 16, 2025). "Orthocell secures A$30 million placement to accelerate US rollout of Remplir." Available at: https://www.proactiveinvestors.com/companies/news/1080542/orthocell-secures-a-30-million-placement-to-accelerate-us-rollout-of-remplir-1080542.html
- Orthocell Official Website. "Remplir - Redefining nerve repair." Available at: https://orthocell.com/remplir/
- HotCopper (September 16, 2025). "Orthocell jumps as nerve repair tech shows hope for erectile dysfunction post-prostate surgery." Available at: https://hotcopper.com.au/news/asx-news/152781/orthocell-jumps-as-nerve-repair-tech-shows-hope-for-erectile-dysfunction-post-prostate-surgery/
Note: This article is for informational purposes only. Patients should consult with their healthcare providers about all treatment options. Remplir™ is not yet FDA-approved specifically for use in prostate surgery nerve protection in the United States, though it has received general FDA clearance for nerve repair applications.


Comments
Post a Comment