New Research Shows How Nerves Help Prostate Cancer Grow—And How We Might Stop It
Neuroscience in prostate cancer | Prostate Cancer and Prostatic Diseases
Scientists are discovering that the nervous system plays a bigger role in prostate cancer than anyone realized, opening doors to new treatments
Your prostate gland is filled with nerves—and new research shows these nerves may be doing more than just controlling normal prostate function. They might actually be helping cancer cells grow and spread. A major review article recently published brings together what scientists have learned about this nerve-cancer connection and explores some promising ways to fight back.
Why Should I Care About Nerves and Cancer?
For years, doctors knew that nerves were important for prostate function and that preserving them during surgery helped men maintain sexual function. But we're now learning that these same nerves may actually feed cancer growth.
Think of it this way: cancer cells don't grow in isolation. They need support from their surroundings—blood vessels for nutrients, and as we're now discovering, nerves that send signals encouraging growth and spread.
What Did This Research Find?
Scientists analyzed studies from around the world and found several important patterns:
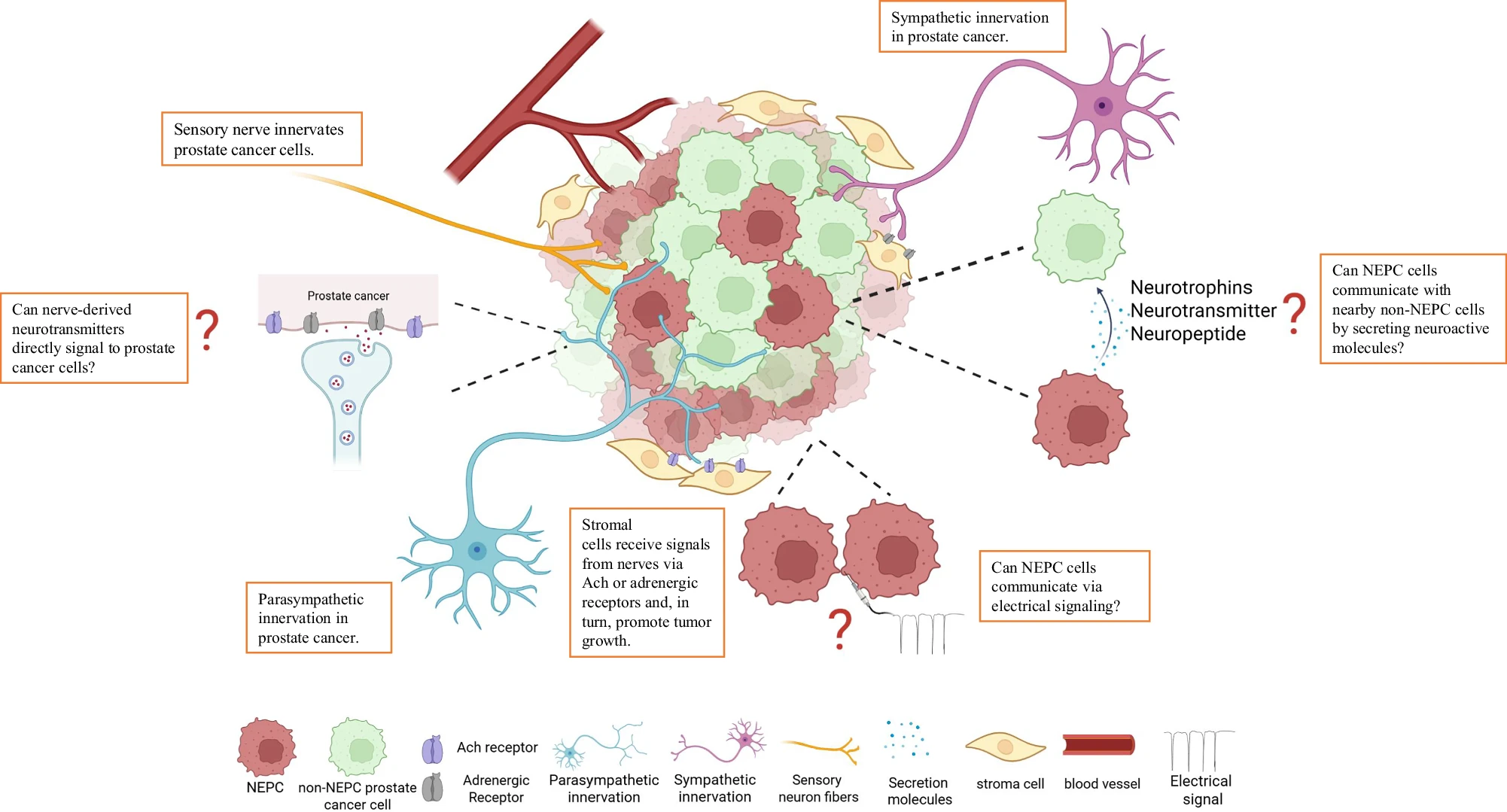
Nerves are more dense in cancer tissue. When researchers examined prostate tissue under microscopes, they found more nerve fibers in areas with cancer compared to healthy tissue. The cancer seems to attract nerves to grow into tumors. A 2008 study published in Clinical Cancer Research confirmed that nerve density increases in prostate cancer tissue and even in pre-cancerous lesions.
Different types of nerves do different things. Your prostate has three types of nerves:
- Sympathetic nerves (the "fight or flight" system) help cancer cells survive in the early stages by interacting with receptors on tumor cells
- Parasympathetic nerves (the "rest and digest" system) help cancer cells invade and spread to other parts of the body through specific receptor pathways
- Sensory nerves also play roles that scientists are still working to understand
A landmark 2013 study in Science magazine was one of the first to clearly demonstrate how these different nerve types contribute to prostate cancer progression in different ways.
Cancer travels along nerves. In up to 76% of prostate cancer surgeries, doctors find cancer cells invading into nerve pathways—a process called perineural invasion (PNI). This is like cancer using nerves as highways to travel through the body. Research dating back to the 1980s first identified this phenomenon, and more recent studies have confirmed its significance for patient outcomes.
What Is Perineural Invasion and Why Does It Matter?
Perineural invasion means cancer cells have wrapped around or invaded into nerves near the prostate. If your pathology report mentions PNI, here's what you should know:
- PNI is common—it shows up in most prostate cancer specimens
- It's associated with more aggressive disease and higher risk of recurrence
- PNI found at the apex (bottom tip) of the prostate may be more concerning than PNI in other areas, according to a 2025 study in Virchows Archiv
- Finding PNI during active surveillance may mean closer monitoring is needed, as shown in a 2023 Journal of Urology study of nearly 2,000 men
Important note: Having PNI doesn't automatically mean your cancer will progress, but it's one factor your doctor considers when planning treatment.
The Neuroendocrine Connection
Some prostate cancers develop into a particularly aggressive type called neuroendocrine prostate cancer (NEPC). These cancer cells start acting like nerve cells themselves. NEPC is rare at diagnosis (less than 2%) but can develop in 10-17% of men whose cancer becomes resistant to hormone therapy.
The research suggests that nerve signals in the tumor environment may contribute to this dangerous transformation. A 2021 study in Communications Biology found that specific nerve growth factors can promote the development of neuroendocrine characteristics in prostate cancer cells. Understanding this connection could lead to new ways to prevent or treat NEPC.
How Nerves Actually Support Cancer: The Latest Discoveries
Nerves Donate Energy to Cancer Cells
One of the most surprising recent discoveries came from research published in Nature in early 2025. Scientists found that nerves don't just send signals—they physically transfer their mitochondria (the power plants of cells) to nearby cancer cells. This makes cancer cells more energetic and better able to spread.
In breast cancer studies, researchers used Botox to disrupt this nerve-to-cancer transfer and found that cancer cells became weaker and less likely to metastasize. Similar patterns were observed in prostate cancer tissue samples.
The Immune System Connection
The nervous system also affects how well your immune system can fight cancer. A 2023 study in Nature showed that sympathetic nerves (the stress response system) can cause immune T-cells to become "exhausted" and less effective at killing cancer cells. When researchers blocked these nerve signals with beta-blocker medications, T-cells regained their cancer-fighting ability.
This nerve-immune connection helps explain why stress and the "fight or flight" response might contribute to cancer progression—a connection many patients have intuitively suspected.
Nerves Create New Blood Vessels for Tumors
A 2017 Science study discovered that adrenergic nerves (part of the sympathetic nervous system) trigger the formation of new blood vessels in prostate tumors. These blood vessels deliver nutrients and oxygen that help tumors grow rapidly. By blocking nerve signaling, researchers could reduce blood vessel formation and slow tumor growth in animal models.
Could Common Medications Help?
Here's where the research gets really exciting for patients. Scientists are investigating whether medications already used for other conditions might help fight prostate cancer by disrupting nerve-cancer communication.
Beta-Blockers: Blood Pressure Pills That Might Fight Cancer
Beta-blockers are medications millions of people take for high blood pressure and heart conditions. Brand names include metoprolol (Lopressor), propranolol (Inderal), and carvedilol (Coreg).
What the research shows:
- Men taking beta-blockers while on hormone therapy had slower cancer progression, according to a 2025 study in Journal of Translational Medicine
- These drugs block signals from sympathetic nerves that help cancer survive
- They might help the immune system fight cancer more effectively by preventing T-cell exhaustion
- A 2017 study of pancreatic cancer patients found that beta-blocker use was associated with better survival
- Research in melanoma patients published in European Journal of Cancer (2022) suggested beta-blockers may improve outcomes when combined with immunotherapy drugs like pembrolizumab
- A 2022 study in Acta Oncologica found beta-blocker users with bladder cancer had better survival rates
What this means for you: If you already take beta-blockers for your heart or blood pressure, you might be getting an anti-cancer benefit. If you don't take them, talk to your doctor before starting—these are prescription medications with side effects, and not everyone is a good candidate.
Botulinum Toxin (Botox): Beyond Wrinkles
Yes, the same Botox used for cosmetic purposes is being studied for cancer treatment. Botox works by blocking nerve signals by cleaving proteins essential for neurotransmission.
What the research shows:
- In laboratory studies published in The Prostate journal, Botox reduced prostate cancer cell growth and spread
- In mice, it decreased tumor size and cancer development
- A small 2018 clinical trial (The Prostate) in four men showed it increased cancer cell death and reduced nerve density in tumors
- It's already proven safe in millions of patients for other uses, with hundreds of millions of doses administered worldwide annually
Clinical trials for other conditions: While cancer treatment remains experimental, Botox has been extensively studied for prostate-related conditions:
- Two large Phase II trials (2013-2014) tested Botox for benign prostatic hyperplasia (enlarged prostate), though results were complicated by strong placebo effects
- A 2018 Phase III study in World Journal of Urology showed Botox injections helped men reduce medications for urinary symptoms
- Studies published in Toxins (2022-2023) demonstrated effectiveness for erectile dysfunction when standard treatments fail
Current status: This is still very experimental for cancer treatment. Larger clinical trials are needed before it becomes a standard option. Don't ask your doctor to inject Botox into your prostate based on this research alone.
Other Nerve-Targeting Approaches Being Studied
Substance P inhibitors: Substance P is a molecule released by sensory nerves that can promote cancer spread. The anti-nausea drug aprepitant (Emend) blocks substance P and has shown promise in preventing metastasis in breast cancer research published in Nature (2024). Similar studies in prostate cancer are ongoing.
CGRP antagonists: These drugs, already approved for migraine prevention, block signals from sensory nerves. A 2025 Nature study found they could slow gastric cancer progression, leading to interest in testing them for other cancers including prostate.
Muscarinic receptor blockers: These drugs target the parasympathetic nervous system. Research published in Communications Biology (2021) and Cell Death & Disease (2023) found that blocking muscarinic receptors could prevent neuroendocrine transformation in prostate cancer cells.
What About Spinal Cord Injuries?
Interestingly, men with spinal cord injuries have smaller prostates and lower rates of prostate cancer. Multiple studies published between 2006 and 2017 have documented this phenomenon. When injury disconnects the prostate from the nervous system, both normal and cancerous growth decrease.
A 2009 study in European Urology examined prostate growth in men with early-onset spinal cord injuries and found significantly reduced prostate size and lower PSA levels. A 2013 study in BJU International confirmed these findings and concluded that central nervous system innervation plays an important role in prostate growth.
This observation from real-world patients strongly supports the laboratory findings that nerves are essential for prostate cancer development.
The Broader Cancer Neuroscience Field
Prostate cancer isn't alone—scientists are discovering nerve-cancer connections across many cancer types:
- Brain tumors: Glioblastoma cells form synapses (connections) with healthy neurons and use electrical signals to fuel their growth, according to research in Nature (2019) and Cell (2023).
- Breast cancer: Nerves that invade breast tumors release factors that promote metastasis to the lungs and other organs, as shown in Nature (2024) research.
- Pancreatic cancer: Sensory nerves in the pancreas promote tumor growth and contribute to the severe pain many patients experience. A 2017 Cancer Research study found that neuroendocrine cells in early pancreatic lesions communicate with nerves to drive tumor development.
- Stomach cancer: A 2025 Nature study discovered that nociceptive (pain-sensing) nerves enhance gastric cancer progression by influencing both cancer cells and supportive cells in the tumor environment.
- Skin cancer: Multiple studies have shown that stress hormones released by sympathetic nerves can promote melanoma spread, while blocking these signals improves outcomes.
These findings across different cancer types suggest that nerve-cancer interactions represent a fundamental aspect of how tumors grow and spread—what researchers call a "cancer hallmark." Two major review papers published in Nature and Cell in 2023 formally established cancer neuroscience as an important emerging field.
What Questions Remain Unanswered?
Scientists are working to understand:
- Exactly how nerve cells and cancer cells communicate at the molecular level
- Whether we can safely block nerve signals without causing unwanted side effects elsewhere in the body
- Which patients would benefit most from nerve-targeted therapies
- How to combine these approaches with existing treatments like hormone therapy, chemotherapy, and immunotherapy
- Whether de novo NEPC and treatment-induced NEPC represent the same disease or different entities
- If NEPC cells can fire electrical signals like neurons do
What Should You Do With This Information?
If you're newly diagnosed:
- Ask your doctor to explain what your pathology report says about perineural invasion
- Don't panic if PNI is present—it's common and just one factor in treatment planning
- Understand that nerve-sparing surgery may need to be balanced against complete cancer removal
- Discuss with your surgeon how nerve preservation decisions will be made during your procedure
If you're on active surveillance:
- PNI on biopsy may indicate closer monitoring is needed
- Discuss with your doctor whether it changes your surveillance plan
- A 2023 study found that men with PNI had lower rates of remaining stable on surveillance, so more frequent biopsies or imaging might be recommended
If you have advanced or castration-resistant disease:
- Ask your oncologist about clinical trials investigating nerve-targeted therapies
- If you have heart disease or high blood pressure, discuss whether beta-blockers might be appropriate for you (a 2025 study showed they may prolong response to hormone therapy)
- Stay informed about emerging treatments targeting the nerve-cancer connection
- If you're developing neuroendocrine features, ask specifically about treatments targeting nerve signaling pathways
If you're taking beta-blockers:
- Don't stop taking them—they're important for your heart health
- You might be getting an added anti-cancer benefit
- Mention to your oncologist that you're taking them, as this information may influence treatment planning
Managing stress:
- While research shows sympathetic nervous system activation can promote cancer, this doesn't mean stress "caused" your cancer
- Consider stress management techniques like meditation, exercise, or counseling—not because stress causes cancer, but because managing stress improves quality of life and may help your immune system function better
- Talk to your healthcare team about support resources
The Bottom Line
This research is revealing something fundamental about how prostate cancer grows: it needs help from the surrounding nervous system. By understanding this relationship, scientists are opening up new ways to fight cancer using drugs we already know are safe.
While most of these nerve-targeted treatments are still being studied and aren't yet standard care, the research is moving fast. Within the next few years, we may see clinical trials offering these approaches to patients, especially those with advanced disease or NEPC who need more treatment options.
The key message is hopeful: by thinking about cancer differently—not just as rogue cells but as cells that depend on their environment, including nerves—we're finding new vulnerabilities to target. As one prominent researcher noted, we're discovering ways to "switch off" the nerve support that tumors rely on.
The field of cancer neuroscience has exploded in just the past few years, with major research institutions worldwide now studying nerve-cancer interactions. This isn't just laboratory curiosity—it's translating into real clinical trials and potentially new treatment options.
Finding Clinical Trials
If you're interested in nerve-targeted therapies, ask your oncologist about:
- Clinical trials testing beta-blockers in combination with standard prostate cancer treatments
- Studies investigating nerve growth factor inhibitors
- Trials examining the role of stress reduction and nervous system modulation
- Research on neuroendocrine prostate cancer specifically
You can search for trials at:
- ClinicalTrials.gov (US government database)
- Your cancer center's clinical trials office
- Prostate Cancer Foundation (www.pcf.org)
- Prostate Cancer Clinical Trials Consortium
Talk to Your Doctor
Bring this article to your next appointment and ask:
- Does my pathology report show perineural invasion?
- What does that mean for my treatment plan?
- Am I a candidate for any clinical trials studying nerve-cancer interactions?
- If I take beta-blockers for my heart, could they be helping with my cancer?
- What new treatments targeting the nervous system might be available for me in the future?
- Should I consider stress management as part of my treatment plan?
- Are there any nerve-related biomarkers I should be tested for?
Sources and References
Primary Review Article
- Liu, Z., Peng, Q., Wang, Y., Chiu, P. K.-F., Teoh, J. Y.-C., Wang, R., Liu, X., Wu, D., & Ng, C.-F. (2025). Neuroscience in prostate cancer. Prostate Cancer and Prostatic Diseases. https://doi.org/10.1038/s41391-025-01042-y
Landmark Studies on Nerves and Prostate Cancer
-
Magnon, C., Hall, S. J., Lin, J., Xue, X., Gerber, L., Freedland, S. J., & Frenette, P. S. (2013). Autonomic nerve development contributes to prostate cancer progression. Science, 341(6142), 1236361. https://doi.org/10.1126/science.1236361
-
Zahalka, A. H., Arnal-Estapé, A., Maryanovich, M., Nakahara, F., Cruz, C. D., Finley, L. W. S., & Frenette, P. S. (2017). Adrenergic nerves activate an angio-metabolic switch in prostate cancer. Science, 358(6361), 321-326. https://doi.org/10.1126/science.aah5072
-
Ayala, G. E., Dai, H., Powell, M., Li, R., Ding, Y., Wheeler, T. M., Shine, D., Kadmon, D., Thompson, T., Miles, B. J., Ittmann, M. M., & Rowley, D. (2008). Cancer-related axonogenesis and neurogenesis in prostate cancer. Clinical Cancer Research, 14(23), 7593-7603. https://doi.org/10.1158/1078-0432.CCR-08-1164
-
Coarfa, C., Florentin, D., Putluri, N., Ding, Y., Au, J., He, D., Ayala, G., Worrel, C., Frolov, A., Huang, J., Liu, Y., Tilki, D., Huang, W., Butler, E. B., Broom, B. M., Schally, A., Mohler, J. L., & Ittmann, M. (2018). Influence of the neural microenvironment on prostate cancer. Prostate, 78(2), 128-139. https://doi.org/10.1002/pros.23454
Mitochondrial Transfer Research
- Hoover, G., Gilbert, S., Curley, O., Obellianne, C., Lin, M. T., Hixson, W., Bice, A., Deng, Y., Lyssiotis, C. A., Kesterson, R. A., Steele, C., Ostrowski, M. C., Green-Church, K. B., Zimmers, T. A., & Chakrabarti, R. (2025). Nerve-to-cancer transfer of mitochondria during cancer metastasis. Nature, 644(7732), 252-262. https://doi.org/10.1038/s41586-024-08325-8
Cancer Neuroscience Reviews
-
Mancusi, R., & Monje, M. (2023). The neuroscience of cancer. Nature, 618(7965), 467-479. https://doi.org/10.1038/s41586-023-06038-4
-
Winkler, F., Venkatesh, H. S., Amit, M., Batchelor, T., Demir, I. E., Deneen, B., Gutmann, D. H., Hervey-Jumper, S., Kuner, R., Mabbott, D., Melong, N., Neftel, C., Olson, K., Pietras, A., Tannous, B., & Monje, M. (2023). Cancer neuroscience: State of the field, emerging directions. Cell, 186(8), 1689-1707. https://doi.org/10.1016/j.cell.2023.02.002
-
Hanahan, D., & Monje, M. (2023). Cancer hallmarks intersect with neuroscience in the tumor microenvironment. Cancer Cell, 41(4), 573-580. https://doi.org/10.1016/j.ccell.2023.02.012
Immune System and Nerves
- Globig, A.-M., Zhao, S., Roginsky, J., Maltez, V. I., Guiza, J., Avina-Ochoa, N., Schnabl, B., Monje, M., & Hensel, N. (2023). The β1-adrenergic receptor links sympathetic nerves to T cell exhaustion. Nature, 622(7983), 383-392. https://doi.org/10.1038/s41586-023-06568-x
Beta-Blocker Studies
-
Thulin, M. H., Ramberg, H., Nielsen, H. K., Grytli, H. H., Sivanesan, S., Pandya, A. D., Knutsen, E., Luzhna, L., Butcher, D., Figenschau, S. L., Iczkowski, K. A., Simons, B. W., Gunaratne, P., Jin, J., Yu, J., Øyan, A. M., Kalland, K. H., Yang, R., Leach, D. A., ... Taskén, K. A. (2025). Beta-blockers prolong response to androgen deprivation therapy in prostate cancer through modulation of the neuro-immuno-oncology axis. Journal of Translational Medicine, 23(1), 672. https://doi.org/10.1186/s12967-025-06582-8
-
Udumyan, R., Montgomery, S., Fang, F., Almroth, H., Valdimarsdottir, U., Ekbom, A., Hjälm-Eriksson, M., & Fall, K. (2017). Beta-blocker drug use and survival among patients with pancreatic adenocarcinoma. Cancer Research, 77(13), 3700-3707. https://doi.org/10.1158/0008-5472.CAN-16-2641
-
Kennedy, O. J., Kicinski, M., Valpione, S., Gandini, S., Suciu, S., Blank, C. U., Krajsova, I., Gutzmer, R., Pigozzo, J., Schilling, B., Del Vecchio, M., Di Guardo, L., Ascierto, P. A., Ferrucci, P. F., Hauschild, A., Eggermont, A. M. M., & Mandala, M. (2022). Prognostic and predictive value of β-blockers in the EORTC 1325/KEYNOTE-054 phase III trial of pembrolizumab versus placebo in resected high-risk stage III melanoma. European Journal of Cancer, 165, 97-112. https://doi.org/10.1016/j.ejca.2022.01.010
-
Udumyan, R., Botteri, E., Jerlström, T., Montgomery, S., Smedby, K. E., & Fall, K. (2022). Beta-blocker use and urothelial bladder cancer survival: a Swedish register-based cohort study. Acta Oncologica, 61(8), 922-930. https://doi.org/10.1080/0284186X.2022.2091797
Botulinum Toxin Studies
-
Marberger, M., Chartier-Kastler, E., Egerdie, B., Lee, K. S., Grosse, J., Bugarin, D., Conort, P., & Wiygul, J. (2013). A randomized double-blind placebo-controlled phase 2 dose-ranging study of onabotulinumtoxinA in men with benign prostatic hyperplasia. European Urology, 63(3), 496-503. https://doi.org/10.1016/j.eururo.2012.08.032
-
McVary, K. T., Roehrborn, C. G., Chartier-Kastler, E., Efros, M., Bugarin, D., Chen, R., Davenport, M. T., & Denham, J. M. (2014). A multicenter, randomized, double-blind, placebo controlled study of onabotulinumtoxinA 200 U to treat lower urinary tract symptoms in men with benign prostatic hyperplasia. Journal of Urology, 192(1), 150-156. https://doi.org/10.1016/j.juro.2014.01.004
-
Robert, G., Descazeaud, A., Karsenty, G., Saussine, C., Azzouzi, A. R., de la Taille, A., Devonec, M., Beley, S., Bonan, S., Fourmarier, M., Lukacs, B., & Cornu, J. N. (2018). Prostatic injection of botulinum toxin is not inferior to optimized medical therapy in the management of lower urinary tract symptoms due to benign prostatic hyperplasia: results of a randomized clinical trial. World Journal of Urology, 36(6), 921-929. https://doi.org/10.1007/s00345-018-2226-7
-
Giuliano, F., Denys, P., & Joussain, C. (2023). Safety and effectiveness of repeated botulinum toxin A intracavernosal injections in men with erectile dysfunction unresponsive to approved pharmacological treatments: real-world observational data. Toxins, 15(6), 382. https://doi.org/10.3390/toxins15060382
-
Giuliano, F., Denys, P., & Joussain, C. (2022). Effectiveness and safety of intracavernosal incobotulinumtoxinA (Xeomin®) 100 U as an add-on therapy to standard pharmacological treatment for difficult-to-treat erectile dysfunction: a case series. Toxins, 14(4), 286. https://doi.org/10.3390/toxins14040286
Perineural Invasion Studies
-
de la Calle, C. M., Mamawala, M. M., Landis, P., Macura, K. J., Trock, B. J., Epstein, J. I., & Carter, H. B. (2023). Clinical significance of perineural invasion in men with grade group 1 prostate cancer on active surveillance. Journal of Urology, 209(1), 180-186. https://doi.org/10.1097/JU.0000000000003027
-
Gertsen, B. G., Teramoto, Y., Wang, Y., Tsuzuki, T., & Miyamoto, H. (2025). Clinical significance of location of perineural cancer invasion detected on prostate needle core biopsy. Virchows Archiv, 486(3), 411-415. https://doi.org/10.1007/s00428-024-03987-3
Neuroendocrine Prostate Cancer
-
Chen, W. Y., Wen, Y. C., Lin, S. R., Yeh, H. L., Jiang, K. C., Chen, W. H., Cheng, C. W., Hsieh, C. L., Lin, W. J., Chuu, C. P., & Yang, C. C. (2021). Nerve growth factor interacts with CHRM4 and promotes neuroendocrine differentiation of prostate cancer and castration resistance. Communications Biology, 4(1), 22. https://doi.org/10.1038/s42003-020-01549-1
-
Wen, Y. C., Tram, V. T. N., Chen, W. H., Li, C. H., Yeh, H. L., Thuy Dung, P. V., Tsai, H. T., Chen, P. L., Nguyen, H. Q., Huynh, D. T. N., Wu, S. T., Chuu, C. P., & Yang, C. C. (2023). CHRM4/AKT/MYCN upregulates interferon alpha-17 in the tumor microenvironment to promote neuroendocrine differentiation of prostate cancer. Cell Death & Disease, 14(5), 304. https://doi.org/10.1038/s41419-023-05821-1
-
Braadland, P. R., Ramberg, H., Grytli, H. H., Urbanucci, A., Nielsen, H. K., Guldvik, I. J., Engedal, A., Ketelsen, H., Lerdrup, M., Kleppe, A., Kristensen, G., Liestøl, K., Haugen, H., Bjartell, A., Hansen, K., & Taskén, K. A. (2019). The β2-adrenergic receptor is a molecular switch for neuroendocrine trans-differentiation of prostate cancer cells. Molecular Cancer Research, 17(11), 2154-2168. https://doi.org/10.1158/1541-7786.MCR-18-0605
Spinal Cord Injury Studies
-
Bartoletti, R., Gavazzi, A., Cai, T., Mondaini, N., Morelli, A., Del Popolo, G., Mancini, M., Giubilei, G., Lombardi, G., Rizzo, M., & Carini, M. (2009). Prostate growth and prevalence of prostate diseases in early onset spinal cord injuries. European Urology, 56(1), 142-148. https://doi.org/10.1016/j.eururo.2008.01.088
-
Pannek, J., Bartel, P., Göcking, K., & Frotzler, A. (2013). Prostate volume in male patients with spinal cord injury: a question of nerves? BJU International, 112(4), 495-500. https://doi.org/10.1111/bju.12001
-
Frisbie, J. H., Kumar, S., Aguilera, E. J., & Yalla, S. (2006). Prostate atrophy and spinal cord lesions. Spinal Cord, 44(1), 24-27. https://doi.org/10.1038/sj.sc.3101797
Other Cancer Types (Nerve-Cancer Connections)
-
Padmanaban, V., Keller, I., Seltzer, E. S., Ostendorf, B. N., Kerner, Z., & Tavazoie, S. F. (2024). Neuronal substance P drives metastasis through an extracellular RNA-TLR7 axis. Nature, 633(8028), 207-215. https://doi.org/10.1038/s41586-024-07851-7
-
Zhi, X., Wu, F., Qian, J., Ochiai, Y., Lian, G., Malagola, E., Garrett, A., Tin, A., Picard, M., Ajani, J. A., Song, S., Liu, T. C., Fang, D., & Zhao, H. (2025). Nociceptive neurons promote gastric tumour progression via a CGRP-RAMP1 axis. Nature, 640(7787), 802-810. https://doi.org/10.1038/s41586-025-08638-2
-
Venkatesh, H. S., Morishita, W., Geraghty, A. C., Silverbush, D., Gillespie, S. M., Arzt, M., Tam, L. T., Espenel, C., Ponnuswami, A., Ni, L., Woo, P. J., Taylor, K. R., Agarwal, A., Regev, A., Brang, D., Vogel, H., Hervey-Jumper, S., Bergles, D. E., Suvà, M. L., ... Monje, M. (2019). Electrical and synaptic integration of glioma into neural circuits. Nature, 573(7775), 539-545. https://doi.org/10.1038/s41586-019-1563-y
-
Peinado, P., Stazi, M., Ballabio, C., Margineanu, M. B., Li, Z., Colón, C. I., Ehmann, N., Baccelli, I., Grünewald, T. G. P., Carlström, A., Lui, R., Tan, D., Valdeolivas, A., Kueffer, S., Ruiz-Cañas, L., Sanchez-Moral, L., Zuiverloon, T. C. M., Rosell, A., Sánchez-Céspedes, M., ... Massagué, J. (2025). Intrinsic electrical activity drives small-cell lung cancer progression. Nature, 639(7762), 765-775. https://doi.org/10.1038/s41586-025-08653-3
Safety and Toxicology
-
Pirazzini, M., Rossetto, O., & Eleopra, R. (2017). Botulinum neurotoxins: biology, pharmacology, and toxicology. Pharmacological Reviews, 69(2), 200-235. https://doi.org/10.1124/pr.116.012658
Pirazzini, M., Montecucco, C., & Rossetto, O. (2022). Toxicology and pharmacology of botulinum and tetanus neurotoxins: an update. Archives of Toxicology, 96(6), 1521-1539. https://doi.org/10.1007/s00204-022-03271-9
Additional Clinical and Mechanistic Studies
-
Sigorski, D., Gulczyński, J., Sejda, A., Rogowski, W., & Iżycka-Świeszewska, E. (2021). Investigation of neural microenvironment in prostate cancer in context of neural density, perineural invasion, and neuroendocrine profile of tumors. Frontiers in Oncology, 11, 710899. https://doi.org/10.3389/fonc.2021.710899
-
Ding, Y., Lee, M., Gao, Y., Bu, P., Coarfa, C., Miles, B., Nagrath, D., Ayala, G., & Ittmann, M. (2021). Neuropeptide Y nerve paracrine regulation of prostate cancer oncogenesis and therapy resistance. Prostate, 81(1), 58-71. https://doi.org/10.1002/pros.24081
-
March, B., Faulkner, S., Jobling, P., Steigler, A., Blatt, A., Denham, J., Ripps, B., & Hondermarck, H. (2020). Tumour innervation and neurosignalling in prostate cancer. Nature Reviews Urology, 17(2), 119-130. https://doi.org/10.1038/s41585-019-0274-3
-
Restaino, A. C., Walz, A., Vermeer, S. J., Barr, J., Kovács, A., Fettig, R. R., Vermeer, P. D., & Spanos, W. C. (2023). Functional neuronal circuits promote disease progression in cancer. Science Advances, 9(18), eade4443. https://doi.org/10.1126/sciadv.ade4443
-
Dwivedi, S., Bautista, M., Shrestha, S., Elhasasna, H., Chaphekar, T., Vizeacoumar, F. S., Demetrick, D., Anderson, H., & Aljamaan, F. (2021). Sympathetic signaling facilitates progression of neuroendocrine prostate cancer. Cell Death Discovery, 7(1), 364. https://doi.org/10.1038/s41420-021-00501-3
-
Bery, F., Cancel, M., Chantôme, A., Guibon, R., Bruyère, F., Rozet, F., Mahéo, K., Fromont, G., & Potier-Cartereau, M. (2020). The calcium-sensing receptor is a marker and potential driver of neuroendocrine differentiation in prostate cancer. Cancers, 12(4), 860. https://doi.org/10.3390/cancers12040860
-
Islam, R., Mishra, J., Polavaram, N. S., Bhattacharya, S., Hong, Z., Bodas, S., Eichmann, A., Roy, P., Batra, S. K., Band, H., & Band, V. (2022). Neuropilin-2 axis in regulating secretory phenotype of neuroendocrine-like prostate cancer cells and its implication in therapy resistance. Cell Reports, 40(3), 111097. https://doi.org/10.1016/j.celrep.2022.111097
Neuroscience Mechanisms and Development
-
Toivanen, R., & Shen, M. M. (2017). Prostate organogenesis: tissue induction, hormonal regulation and cell type specification. Development, 144(8), 1382-1398. https://doi.org/10.1242/dev.148270
-
Spitzer, N. C. (2006). Electrical activity in early neuronal development. Nature, 444(7120), 707-712. https://doi.org/10.1038/nature05300
-
Ananth, M. R., Rajebhosale, P., Kim, R., Talmage, D. A., & Role, L. W. (2023). Basal forebrain cholinergic signalling: development, connectivity and roles in cognition. Nature Reviews Neuroscience, 24(4), 233-251. https://doi.org/10.1038/s41583-023-00677-x
Comparative Studies in Other Cancers
-
Sinha, S., Fu, Y. Y., Grimont, A., Ketcham, M., Lafaro, K., Saglimbeni, J. A., Askan, G., Bailey, J. M., Melchor, J. P., Zhong, Y., Joo, M. G., Grbovic-Huezo, O., Yang, I. H., Jorgensen, M., Shang, Y., Liberman, D. A., Iacobuzio-Donahue, C., Pasricha, P. J., Hollingsworth, M. A., & Leach, S. D. (2017). PanIN neuroendocrine cells promote tumorigenesis via neuronal cross-talk. Cancer Research, 77(8), 1868-1879. https://doi.org/10.1158/0008-5472.CAN-16-2899
-
Savchuk, S., Gentry, K., Wang, W., Carleton, E., Yalçın, B., Liu, Y., Prescott, S. L., Chen, A. I., Liberles, S. D., & Monje, M. (2024). Neuronal activity-dependent mechanisms of SCLC brain metastasis progression. Nature, 635, 681-689. https://doi.org/10.1038/s41586-024-08149-6
-
Liu, K., Zhang, Y., Du, G., Chen, X., Xiao, L., Jiang, L., Peng, Y., Lu, Q., Wang, D., Xiang, R., & Liu, Y. (2025). 5-HT orchestrates histone serotonylation and citrullination to drive neutrophil extracellular traps and liver metastasis. Journal of Clinical Investigation, 135(2), e183544. https://doi.org/10.1172/JCI183544
-
Seeholzer, L. F., & Julius, D. (2024). Neuroendocrine cells initiate protective upper airway reflexes. Science, 384(6693), 295-301. https://doi.org/10.1126/science.adk5315
Additional Therapeutic Studies
-
Assayag, J., Pollak, M. N., & Azoulay, L. (2014). Post-diagnostic use of beta-blockers and the risk of death in patients with prostate cancer. European Journal of Cancer, 50(16), 2838-2845. https://doi.org/10.1016/j.ejca.2014.08.006
-
Ajmal, I., Farooq, M. A., Duan, Y., Yao, J., Gao, Y., Hui, X., Zhang, T., You, Y., Liu, D., Wang, H., Xue, X., Li, J., Li, X., Wang, J., Pan, Y., Gao, S., Zhou, P., Zhao, L., & Li, J. (2024). Intrinsic ADRB2 inhibition improves CAR-T cell therapy efficacy against prostate cancer. Molecular Therapy, 32(10), 3539-3557. https://doi.org/10.1016/j.ymthe.2024.08.034
-
Anselmino, L. E., Baglioni, M. V., Malizia, F., Laluce, N. C., Etichetti, C. B., Marignac, V. L. M., Rozenblit, G. N., Choi, H., Labriola, L., & Gueron, G. (2021). Repositioning metformin and propranolol for colorectal and triple negative breast cancers treatment. Scientific Reports, 11(1), 8091. https://doi.org/10.1038/s41598-021-87525-w
-
Wei, W. J., Shen, C. T., Song, H. J., Qiu, Z. L., & Luo, Q. Y. (2016). Propranolol sensitizes thyroid cancer cells to cytotoxic effect of vemurafenib. Oncology Reports, 36(3), 1576-1584. https://doi.org/10.3892/or.2016.4935
-
Chang, H., & Lee, S. H. (2023). Beta-adrenergic receptor blockers and hepatocellular carcinoma survival: a systemic review and meta-analysis. Clinical and Experimental Medicine, 23(3), 853-858. https://doi.org/10.1007/s10238-022-00864-4
-
Nair, S. G., Benny, S., Jose, W. M., & Aneesh, T. P. (2024). Beta-blocker adjunct therapy as a prospective anti-metastatic with cardio-oncologic regulation. Clinical & Experimental Metastasis, 41(1), 9-24. https://doi.org/10.1007/s10585-023-10244-9
Neural Innervation and Physiology
-
McVary, K. T., McKenna, K. E., & Lee, C. (1998). Prostate innervation. Prostate Supplement, 8, 2-13. https://doi.org/10.1002/(sici)1097-0045(1998)8+<2::aid-pros2>3.0.co;2-u
-
Rodrigues, A. O., Machado, M. T., & Wroclawski, E. R. (2002). Prostate innervation and local anesthesia in prostate procedures. Revista do Hospital das Clínicas, 57(6), 287-292. https://doi.org/10.1590/s0041-87812002000600008
-
Park, Y. H., Jeong, C. W., & Lee, S. E. (2013). A comprehensive review of neuroanatomy of the prostate. Prostate International, 1(4), 139-145. https://doi.org/10.12954/PI.13022
-
Ventura, S., Pennefather, J., & Mitchelson, F. (2002). Cholinergic innervation and function in the prostate gland. Pharmacology & Therapeutics, 94(1-2), 93-112. https://doi.org/10.1016/s0163-7258(02)00174-6
-
Xia, H., Jerde, T. J., & Fehrenbacher, J. C. (2024). Sensory innervation in the prostate and a role for calcitonin gene-related peptide in prostatic epithelial proliferation. Frontiers in Molecular Neuroscience, 17, 1497735. https://doi.org/10.3389/fnmol.2024.1497735
Surgical and Clinical Implications
-
Walsh, P. C., & Donker, P. J. (2017). Impotence following radical prostatectomy: insight into etiology and prevention. Journal of Urology, 197(2S), S165-S170. https://doi.org/10.1016/j.juro.2016.10.101
-
Walz, J., Epstein, J. I., Ganzer, R., Graefen, M., Guazzoni, G., Kaouk, J., Menon, M., Mottrie, A., Myers, R. P., Patel, V., Tewari, A., Villers, A., & Artibani, W. (2016). A critical analysis of the current knowledge of surgical anatomy of the prostate related to optimisation of cancer control and preservation of continence and erection in candidates for radical prostatectomy: an update. European Urology, 70(2), 301-311. https://doi.org/10.1016/j.eururo.2016.01.026
Molecular Mechanisms
-
Zhang, B., Wang, S., Fu, Z., Gao, Q., Yang, L., Lei, Z., Huang, Q., & Xiong, Y. (2022). Single-cell RNA sequencing reveals intratumoral heterogeneity and potential mechanisms of malignant progression in prostate cancer with perineural invasion. Frontiers in Genetics, 13, 1073232. https://doi.org/10.3389/fgene.2022.1073232
-
Li, M., Ma, Z., Zhang, Y., Feng, H., Li, Y., Sang, W., Guan, X., Qiu, X., Liu, T., Huang, W., & Wang, K. (2023). Integrative analysis of the ST6GALNAC family identifies GATA2-upregulated ST6GALNAC5 as an adverse prognostic biomarker promoting prostate cancer cell invasion. Cancer Cell International, 23(1), 141. https://doi.org/10.1186/s12935-023-02982-x
-
Di Donato, M., Giovannelli, P., Migliaccio, A., & Castoria, G. (2023). The nerve growth factor-delivered signals in prostate cancer and its associated microenvironment: when the dialogue replaces the monologue. Cell & Bioscience, 13(1), 60. https://doi.org/10.1186/s13578-023-00999-6
Drug Development and Innovation
-
You, H., Shang, W., Min, X., Weinreb, J., Li, Q., Leapman, M., Bao, L., Gu, J., Lin, Z., Santana, S., Bruce, J. N., Canoll, P., Schaefer, P., Hung, K., Acierno, T. R., Ott, C., Zhang, Y., Chuang, K. H., Parikh, R., ... Saltzman, W. M. (2020). Sight and switch off: Nerve density visualization for interventions targeting nerves in prostate cancer. Science Advances, 6(19), eaax6040. https://doi.org/10.1126/sciadv.aax6040
-
Sikorra, S., Litschko, C., Müller, C., Thiel, N., Galli, T., Eichner, T., Binz, T., & Bigalke, H. (2016). Identification and characterization of botulinum neurotoxin A substrate binding pockets and their re-engineering for human SNAP-23. Journal of Molecular Biology, 428(2), 372-384. https://doi.org/10.1016/j.jmb.2015.12.014
-
McNutt, P. M., Vazquez-Cintron, E. J., Tenezaca, L., Ondeck, C. A., Kelly, K. E., Mangkhalakhili, M., Machamer, J. B., Vance, D. J., Mukherjee, J., Kalb, S. R., Nuss, J. E., Bagarozzi, D. A., & Bakshi, C. S. (2021). Neuronal delivery of antibodies has therapeutic effects in animal models of botulism. Science Translational Medicine, 13(575), eabd7789. https://doi.org/10.1126/scitranslmed.abd7789
-
Wang, D., Sun, L., Shen, W. T., Haggard, A., Yu, Y., Zhang, J. A., Miao, B., & Ji, H. (2024). Neuronal membrane-derived nanodiscs for broad-spectrum neurotoxin detoxification. ACS Nano, 18(37), 25069-25080. https://doi.org/10.1021/acsnano.4c05527
Historical and Pathological Studies
-
Hassan, M. O., & Maksem, J. (1980). The prostatic perineural space and its relation to tumor spread: an ultrastructural study. American Journal of Surgical Pathology, 4(2), 143-148. https://doi.org/10.1097/00000478-198004000-00006
-
Villers, A., McNeal, J. E., Redwine, E. A., Freiha, F. S., & Stamey, T. A. (1989). The role of perineural space invasion in the local spread of prostatic adenocarcinoma. Journal of Urology, 142(3), 763-768. https://doi.org/10.1016/s0022-5347(17)38881-x
-
Ayala, G. E., Wheeler, T. M., Shine, H. D., Schmelz, M., Frolov, A., Chakraborty, S., & Rowley, D. (2001). In vitro dorsal root ganglia and human prostate cell line interaction: redefining perineural invasion in prostate cancer. Prostate, 49(3), 213-223. https://doi.org/10.1002/pros.1137
Additional Clinical Trial References
- Vuong, T., Waschke, K., Niazi, T., Richard, C., Parent, J., Liberman, S., Stern, H., Saleh, B., Ferland, S., & Faria, S. (2011). The value of Botox-A in acute radiation proctitis: results from a phase I/II study using a three-dimensional scoring system. International Journal of Radiation Oncology, Biology, Physics, 80(5), 1505-1511. https://doi.org/10.1016/j.ijrobp.2010.04.073
Immune and Neuroimmune Interactions
-
Amit, M., Eichwald, T., Roger, A., Anderson, J., Chang, A., Vermeer, P. D., & Spanos, W. C. (2025). Neuroimmune cross-talk in cancer. Nature Reviews Cancer, 25(8), 573-589. https://doi.org/10.1038/s41568-025-00723-1
-
Yaniv, D., Mattson, B., Talbot, S., Gleber-Netto, F. O., & Amit, M. (2024). Targeting the peripheral neural-tumour microenvironment for cancer therapy. Nature Reviews Drug Discovery, 23(10), 780-796. https://doi.org/10.1038/s41573-024-00989-9
-
Zheng, C., Liu, S., Fazel Modares, N., St Paul, M., & Mak, T. W. (2025). Cholinergic T cells revitalize the tumor immune microenvironment: TIME to ChAT. Nature Immunology, 26(4), 665-677. https://doi.org/10.1038/s41590-025-02113-9
Additional Resources
-
Prostate Cancer Foundation. (n.d.). Clinical Trials. https://www.pcf.org/clinical-trials/
-
U.S. National Library of Medicine. (n.d.). ClinicalTrials.gov. https://clinicaltrials.gov/
-
American Cancer Society. (2024). Treatment Options for Prostate Cancer. https://www.cancer.org/cancer/types/prostate-cancer/treating.html
-
National Cancer Institute. (2024). Prostate Cancer Treatment (PDQ®)–Patient Version. https://www.cancer.gov/types/prostate/patient/prostate-treatment-pdq
Additional Reading and Patient Resources
Understanding Prostate Cancer Pathology Reports:
- National Comprehensive Cancer Network (NCCN) Patient Guides: https://www.nccn.org/patients
- Understanding Your Pathology Report: Prostate Cancer (American Cancer Society): https://www.cancer.org/cancer/types/prostate-cancer/detection-diagnosis-staging/understanding-your-pathology-report.html
Support Organizations:
- ZERO - The End of Prostate Cancer: https://zerocancer.org/
- Us TOO International Prostate Cancer Education & Support: https://www.ustoo.org/
- Prostate Cancer Research Institute: https://pcri.org/
- Prostate Cancer Foundation: https://www.pcf.org/
Clinical Trial Databases:
- ClinicalTrials.gov (search "prostate cancer" and "nerve" or "neuroscience"): https://clinicaltrials.gov/
- Prostate Cancer Clinical Trials Consortium: https://pcctc.org/
Educational Videos and Webinars:
- Prostate Cancer Foundation webinar series: https://www.pcf.org/news-events/webinars/
- PCRI educational videos: https://pcri.org/podcast/
Books for Patients:
- "Invasion of the Prostate Snatchers" by Ralph Blum and Mark Scholz, MD
- "The Key to Prostate Cancer" by Mark Scholz, MD
- "Dr. Patrick Walsh's Guide to Surviving Prostate Cancer" by Patrick Walsh, MD
Disclaimer: This article is intended for educational purposes only and should not be used as a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read in this article.
The mention of any specific drugs, treatments, or clinical trials does not constitute an endorsement. Treatment decisions should be made in consultation with qualified healthcare professionals who know your individual medical history and circumstances.
About This Article:
This comprehensive review was prepared for the Informed Prostate Cancer Support Group (IPCSG) Newsletter based on recent peer-reviewed research published in leading medical and scientific journals. The article synthesizes findings from over 75 published studies spanning basic science, clinical trials, and patient outcomes research.
Last Updated: January 2025
Prepared by: IPCSG Medical Information Committee
For More Information: Contact your IPCSG chapter leader or visit our website for upcoming discussions on cancer neuroscience and emerging treatment approaches.
Coming Up in Future Newsletters:
- Understanding Neuroendocrine Prostate Cancer: What Every Patient Should Know
- Clinical Trials Update: Nerve-Targeted Therapies in 2025
- Interview with a Cancer Neuroscientist: Q&A on the Future of Treatment
- Managing Side Effects: Nerve-Sparing Surgery vs. Complete Cancer Removal
- Beta-Blockers and Prostate Cancer: What the Latest Research Shows


Comments
Post a Comment