New Target, New Hope: STEAP1 Therapies Show Promise for Advanced Prostate Cancer
STEAP1-targeted strategies in advanced prostate cancer: a review on therapeutic and diagnostic implications | Prostate Cancer and Prostatic Diseases
Multiple clinical trials now recruiting patients as research validates novel protein as effective treatment target
Men with advanced prostate cancer have a new reason for optimism as multiple clinical trials targeting a protein called STEAP1 are now actively recruiting patients, offering access to cutting-edge therapies that are showing encouraging early results.
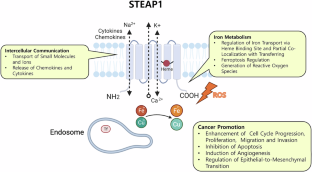
STEAP1 (Six transmembrane epithelial antigen of prostate 1) is highly expressed in over 85% of prostate tumors while showing minimal presence in normal tissues, making it an ideal target for precision cancer treatment. Unlike some other prostate cancer targets, STEAP1 expression remains uniform even as the disease progresses, suggesting it could benefit the majority of patients with metastatic castration-resistant prostate cancer (mCRPC).
Bispecific Antibody Shows Strong Clinical Activity
The most advanced therapy currently in clinical testing is xaluritamig (AMG 509), a bispecific T-cell engager that acts as a bridge between immune cells and cancer cells. In a phase 1 study of 97 heavily pretreated patients with mCRPC, xaluritamig achieved a 49% PSA response rate and a 24% objective response rate—results that exceeded many established treatments.
Based on these promising results, a phase 3 trial called XALute is now underway, comparing xaluritamig to standard treatments in patients with post-taxane mCRPC. The phase 1 trial (NCT04221542) continues to recruit patients at sites including UCSF and other major cancer centers across the United States.
While 72% of patients experienced cytokine release syndrome, most cases were manageable and only two were grade 3. Patients typically require hospitalization for the first doses to monitor for these immune-related side effects.
CAR-T Therapy Advances to Human Testing
One of the most innovative approaches involves engineering a patient's own immune cells to attack cancer. A phase 1/2 clinical trial (NCT06236139) is now recruiting patients to test STEAP1 CAR-T cells combined with enzalutamide, a standard hormone therapy. This trial is being conducted at Fred Hutchinson Cancer Center and other sites.
Preclinical research showed that STEAP1 is expressed more uniformly across tumor cells from advanced prostate cancer than the more commonly explored target PSMA, and the engineered CAR-T cells demonstrated significant antitumor activity in animal models without serious toxicity.
An even more advanced version of this therapy combines STEAP1 CAR-T cells with a collagen-binding IL-12 protein that concentrates immune-stimulating signals directly within tumors while minimizing systemic toxicity—a common limitation of previous IL-12 therapies.
Next-Generation Antibody-Drug Conjugates Enter Trials
Learning from earlier setbacks with STEAP1-targeted therapies, pharmaceutical companies have developed more sophisticated antibody-drug conjugates (ADCs) that deliver chemotherapy directly to cancer cells.
ADRX-0405, developed by Adcentrx Therapeutics, dosed its first patient in January 2025 in a phase 1a/b trial (NCT06710379) for patients with advanced solid tumors including mCRPC. This ADC uses a novel topoisomerase inhibitor payload with a drug-to-antibody ratio of 8, and preclinical studies showed an 83% overall response rate in patient-derived tumor models. Initial clinical data is expected in late 2025.
The original STEAP1 ADC, DSTP3086S, faced challenges with limited efficacy and toxicity. However, these newer designs incorporate lessons learned, including improved linkers, different payloads, and enhanced stability.
What This Means for Patients
For men facing advanced prostate cancer, particularly those who have exhausted standard treatment options, these trials represent genuine opportunities to access promising new therapies.
Who May Be Eligible:
- Men with metastatic castration-resistant prostate cancer
- Those who have received at least one or two prior lines of therapy
- Patients with measurable disease and adequate organ function
- Castrate testosterone levels (with or without ongoing hormone therapy)
Key Trial Identifiers:
- Xaluritamig: NCT04221542 (Phase 1, recruiting)
- STEAP1 CAR-T + enzalutamide: NCT06236139 (Phase 1/2, recruiting)
- ADRX-0405 ADC: NCT06710379 (Phase 1a/b, recruiting)
The Road Ahead
Molecular imaging using 89Zr-DFO-MSTP2109A, a STEAP1-targeting PET tracer, has shown excellent uptake in both bone and soft-tissue metastases, suggesting it could help identify which patients are most likely to benefit from STEAP1-targeted therapies.
Given that STEAP1 expression is not uniform across all patients, companion biomarkers will likely play an important role in selecting the right patients for these therapies.
The diversity of approaches targeting STEAP1—from bispecific antibodies to CAR-T cells to ADCs—means that if one strategy doesn't work for a particular patient, alternatives may be available. This represents a significant shift from the limited options that existed just a few years ago.
How to Learn More
Patients interested in these trials should discuss options with their oncologists. Trial information is available at clinicaltrials.gov, and many major cancer centers are participating sites. Given that these are early-phase trials with limited enrollment, interested patients should inquire sooner rather than later.
Sources
-
Heo, J.Y., Sheng, M., Khalaf, D., Leong, H.S., & Emmenegger, U. (2025). STEAP1-targeted strategies in advanced prostate cancer: a review on therapeutic and diagnostic implications. Prostate Cancer and Prostatic Diseases. https://doi.org/10.1038/s41391-025-00916-4
-
Kelly, W.K., Danila, D.C., Lin, C.C., et al. (2024). Xaluritamig, a STEAP1 × CD3 XmAb 2+1 Immune Therapy for Metastatic Castration-Resistant Prostate Cancer: Results from Dose Exploration in a First-in-Human Study. Cancer Discovery, 14(1), 76-89. https://aacrjournals.org/cancerdiscovery/article/14/1/76/732544
-
Bhatia, V., et al. (2023). Targeting advanced prostate cancer with STEAP1 chimeric antigen receptor T cell and tumor-localized IL-12 immunotherapy. Nature Communications, 14, 2041. https://www.nature.com/articles/s41467-023-37874-2
-
Sasaki, K., Bhatia, V., Asano, Y., et al. (2025). Collagen-binding IL-12-armoured STEAP1 CAR-T cells reduce toxicity and treat prostate cancer in mouse models. Nature Biomedical Engineering. https://doi.org/10.1038/s41551-025-01508-3
-
Flavell, R. (2025). Beyond PSMA in Prostate Cancer: STEAP1, DLL3, and CD46. PSMA and Beyond 2025 Conference. https://www.urotoday.com/conference-highlights/2025-ucsf-ucla-psma-conference/159346
-
Adcentrx Therapeutics. (2025). First Patient Dosed in Phase 1a/b Study of ADRX-0405. Press release, January 6, 2025. https://www.adcentrx.com/adcentrx-therapeutics-doses-first-patient-in-phase-1a-with-adrx-0405-steap1-adc/
-
Fred Hutchinson Cancer Center. (2025). Cell Therapy (STEAP1 CART) With Enzalutamide for the Treatment of Patients With Metastatic Castration-Resistant Prostate Cancer. Clinical trial NCT06236139. https://www.fredhutch.org/en/research/clinical-trials/trial-details.fh_trial_id_15047
-
Morris, M.J., et al. (2025). Trial in progress (XALute): Phase 3 study of xaluritamig vs investigator's choice in post-taxane mCRPC. Journal of Clinical Oncology, 43(16_suppl), TPS5118. https://ascopubs.org/doi/10.1200/JCO.2025.43.16_suppl.TPS5118
-
University of California, San Francisco. (2025). AMG 509 in Participants With Metastatic Castration-Resistant Prostate Cancer. Clinical trial NCT04221542. https://clinicaltrials.ucsf.edu/trial/NCT04221542
-
Zarrabi, K.K., et al. (2025). Molecular characterization of STEAP1 and -2 in advanced prostate cancer. Journal of Clinical Oncology, 43(16_suppl), 5072. https://ascopubs.org/doi/10.1200/JCO.2025.43.16_suppl.5072
-
Shahmoradgoli, M., et al. (2025). Preclinical characterization of a novel STEAP1 antibody-drug conjugate ADRX-0405 for the treatment of mCRPC. Cancer Research, 85(8_Supplement_1), 1159. https://aacrjournals.org/cancerres/article/85/8_Supplement_1/1159/754922
This article is for informational purposes only and does not constitute medical advice. Patients should consult with their healthcare providers about treatment options and clinical trial eligibility.


Comments
Post a Comment