Pluvicto in First-line Hormone-Sensitive Prostate Cancer?
Pluvicto in First-line Hormone-Sensitive Prostate Cancer?
New Treatment Shows Promise for Men with Newly Diagnosed Metastatic Prostate Cancer
PSMAddition Trial Results Presented at ESMO 2025
What You Need to Know: A major clinical trial has shown that adding a targeted radiation therapy called Pluvicto (lutetium-177 PSMA-617) to standard hormone treatment can slow disease progression in men newly diagnosed with metastatic hormone-sensitive prostate cancer. However, experts are debating whether the benefits outweigh the added side effects until more data becomes available.
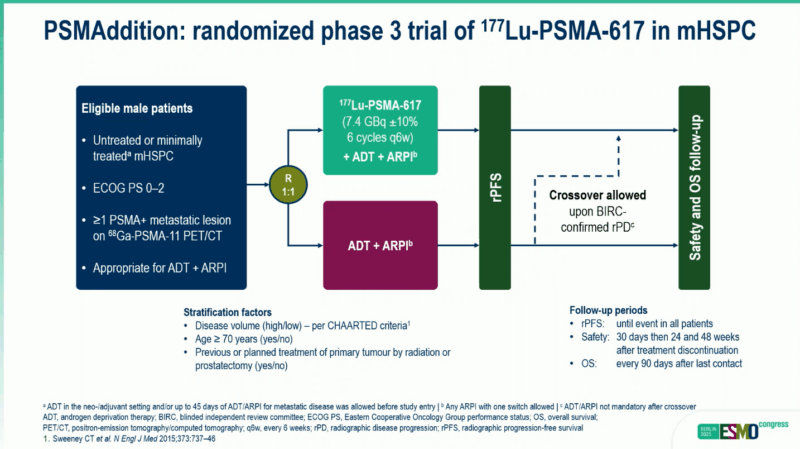
Understanding the Study
The PSMAddition trial enrolled 1,144 men with newly diagnosed or minimally treated metastatic hormone-sensitive prostate cancer whose tumors tested positive for PSMA (prostate-specific membrane antigen). Participants received either standard hormone therapy alone (ADT plus an ARPI such as abiraterone, enzalutamide, apalutamide, or darolutamide) or the same hormone therapy plus Pluvicto administered every 6 weeks for up to 6 cycles.
The Results
After approximately 2 years of follow-up, the trial met its primary goal, showing that adding Pluvicto reduced the risk of disease progression or death by 28%. Dr. Scott Tagawa from NewYork-Presbyterian/Weill Cornell Medical Center, who presented the findings at the European Society for Medical Oncology (ESMO) Congress 2025 in Berlin, noted that the benefits were consistent across all patient subgroups.
Additional benefits included:
- Better PSA control (87% vs 75% of patients achieving PSA levels below 0.2 ng/mL at 48 weeks)
- Longer time to PSA progression and longer time to progression to castration-resistant disease
- A positive trend in overall survival, though the data remains immature with confidence intervals crossing 1
The Trade-offs: Side Effects and Quality of Life
Side effects were more common with Pluvicto—89.4% of patients experienced treatment-related side effects compared to 69.7% without Pluvicto. Most side effects were grade 1 or 2 and included dry mouth, fatigue, dry eyes, and nausea. More serious grade 3 or higher adverse events occurred in 50.7% of patients receiving Pluvicto plus standard therapy compared to 43% on standard therapy alone.
Importantly, patients receiving Pluvicto experienced a numerically shorter time to worsening quality of life—a median of 11.33 months compared to 17.12 months.
Expert Perspectives: Not Everyone is Convinced
Dr. Arun Azad from Peter MacCallum Cancer Center in Melbourne, Australia, who served as the study discussant, expressed significant concerns about implementing this treatment widely at this time. He emphasized that the ultimate goal of cancer treatment is to help patients live longer and live better, and said this goal has not yet been achieved in PSMAddition.
Dr. Azad raised three key questions:
- Will it improve overall survival? With only 47% of overall survival data available and 60% of control arm patients crossing over to receive Pluvicto after progression, it remains unclear whether early use will ultimately extend life
- What about long-term toxicity? Chronic grade 1-2 dry mouth and gastrointestinal toxicity can impair quality of life even if they don't reach grade 3 severity
- Can we identify which patients benefit most? Optimizing patient selection to identify those most likely to benefit from upfront Pluvicto will be crucial
What This Means for Patients
This is the third positive Phase III trial for Pluvicto, following the VISION and PSMAfore studies that led to FDA approval for use in metastatic castration-resistant prostate cancer. Novartis plans to submit these data to regulatory authorities by the end of 2025, and if approved, this could potentially double the number of patients eligible for Pluvicto treatment.
Approximately 172,000 men are diagnosed with metastatic hormone-sensitive prostate cancer each year across major markets including the US, China, Japan, and several European countries. Most of these patients progress to castration-resistant disease, typically within 20 months, which is associated with significantly worse outcomes.
The Bottom Line
The PSMAddition trial represents an important advance in bringing targeted radiation therapy earlier in the prostate cancer treatment journey. While the progression-free survival benefit is clear and statistically significant, the debate among experts highlights that not all positive trials automatically translate to changes in clinical practice.
As Dr. Azad noted, we are moving into an era of personalized medicine where better identifying predictive biomarkers will help use this targeted therapy more effectively.
For now, patients and their doctors should:
- Stay informed as more mature data becomes available
- Discuss individual risk-benefit profiles
- Consider participation in clinical trials when appropriate
- Wait for regulatory review and approval decisions expected later in 2025
The prostate cancer community eagerly awaits the mature overall survival data, which will ultimately determine whether this approach becomes a new standard of care for newly diagnosed metastatic disease.
Sources
-
Brooks, M. (2025). Pluvicto in First-line Hormone-Sensitive Prostate Cancer? Medscape. https://www.medscape.com/viewarticle/pluvicto-first-line-hormone-sensitive-prostate-cancer
-
Novartis. (October 19, 2025). PSMAddition data show Novartis Pluvicto™ delays progression to end-stage prostate cancer [Press release]. https://www.novartis.com/news/media-releases/psmaddition-data-show-novartis-pluvictotm-delays-progression-end-stage-prostate-cancer
-
Novartis. (June 2, 2025). Novartis Pluvicto™ demonstrates statistically significant and clinically meaningful rPFS benefit in patients with PSMA-positive metastatic hormone-sensitive prostate cancer [Press release]. https://www.novartis.com/news/media-releases/novartis-pluvictotm-demonstrates-statistically-significant-and-clinically-meaningful-rpfs-benefit-patients-psma-positive-metastatic-hormone-sensitive-prostate-cancer
-
Pharmaceutical Technology. (October 2025). Novartis's Pluvicto delays progression to end-stage for mHSPC. https://www.pharmaceutical-technology.com/analyst-comment/esmo-2025-novartis-pluvicto-end-stage-mhspc/
-
Oncology News Central. (October 2025). Despite Positive Data From the PSMAddition Trial, Expert at ESMO 2025 Raises Concerns. https://www.oncologynewscentral.com/prostate-cancer/despite-positive-data-expert-raises-concerns-over-prostate-cancer-treatment
-
Tagawa, S.T., et al. (2025). Phase 3 trial of [177Lu]Lu-PSMA-617 combined with ADT + ARPI in patients with PSMA-positive metastatic hormone-sensitive prostate cancer (PSMAddition). Presented at: ESMO Congress 2025; October 17-21, 2025; Berlin, Germany. Abstract LBA6.
-
UroToday. (October 2025). ESMO 2025: Phase III Trial of [177Lu]Lu-PSMA-617 Combined with ADT + ARPI in Patients with PSMA-Positive Metastatic Hormone-Sensitive Prostate Cancer (PSMAddition). https://www.urotoday.com/conference-highlights/esmo-2025/esmo-2025-prostate-cancer/164097-esmo-2025-phase-iii-trial-of-177lu-lu-psma-617-combined-with-adt-arpi-in-patients-with-psma-positive-metastatic-hormone-sensitive-prostate-cancer-psmaddition.html
-
UroToday. (October 2025). ESMO 2025: Discussant – Phase III Trial of [177Lu]Lu-PSMA-617 Combined with ADT + ARPI in Patients with PSMA-Positive Metastatic Hormone-Sensitive Prostate Cancer (PSMAddition). https://www.urotoday.com/conference-highlights/esmo-2025/esmo-2025-prostate-cancer/164134-esmo-2025-discussant-phase-iii-trial-of-177lu-lu-psma-617-combined-with-adt-arpi-in-patients-with-psma-positive-metastatic-hormone-sensitive-prostate-cancer-psmaddition.html
-
ESMO Daily Reporter. (October 2025). Triple combination with radioligand therapy improves radiographic progression-free survival in metastatic prostate cancer. https://dailyreporter.esmo.org/esmo-congress-2025/genitourinary-cancers/triple-combination-with-radioligand-therapy-improves-radiographic-progression-free-survival-in-metastatic-prostate-cancer
-
OncoDaily. (October 2025). PSMAddition: ¹⁷⁷Lu-PSMA-617 Extends PFS in mHSPC. https://oncodaily.com/oncolibrary/psmaddition-%C2%B9%E2%81%B7%E2%81%B7lu-psma-617-mhspc
-
Sartor, O., de Bono, J., Chi, K.N., et al. (2021). Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. New England Journal of Medicine, 385(12), 1091-1103. doi:10.1056/NEJMoa2107322
-
Morris, M., Castellano, D., Herrmann, K., et al. (2024). 177Lu-PSMA-617 versus a change of androgen receptor pathway inhibitor therapy for taxane-naive patients with progressive metastatic castration-resistant prostate cancer (PSMAfore): a phase 3, randomised, controlled trial. The Lancet. doi:10.1016/S0140-6736(24)01653-2
-
ClinicalTrials.gov. (2025). An International Prospective Open-label, Randomized, Phase III Study Comparing 177Lu-PSMA-617 in Combination With SoC, Versus SoC Alone, in Adult Male Patients With mHSPC (PSMAddition). NCT04720157. https://clinicaltrials.gov/study/NCT04720157
For questions about this trial or whether you might be a candidate for Pluvicto, please consult with your oncologist or contact your treatment center.

Comments
Post a Comment