5 Tesla MRI Delivers Sharper Prostate Cancer Images Revealing More Cancers
5 T versus 3 T MRI for prostate cancer: an intra-individual prospective comparison of image quality and diagnostic performance | Prostate Cancer and Prostatic Diseases
But $10 Million Price Tag Raises Questions About Clinical Value
BLUF (Bottom Line Up Front): A January 2026 study shows ultra-high-field 5 Tesla MRI scanners detect prostate cancer more accurately than standard 3 Tesla systems, with 67% stronger signals producing clearer tumor boundaries and better visualization of critical anatomy. However, with fewer than ten 5T scanners worldwide, $7-10 million price tags versus $2-3 million for 3T systems, and questions about whether improved images translate to better patient outcomes, the technology faces a long road from laboratory breakthrough to routine clinical care.
The Promise: Significantly Better Images
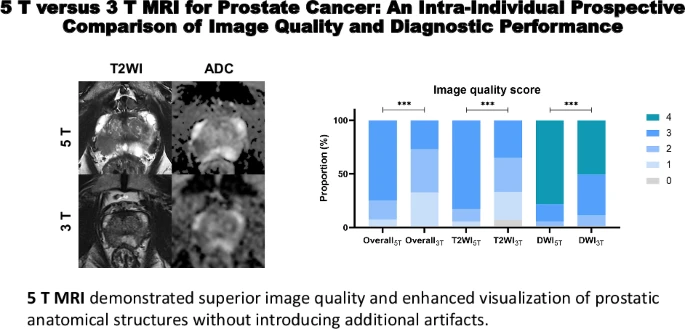
Researchers at West China Hospital of Sichuan University directly compared both technologies in 67 consecutive patients with suspected prostate cancer, having each man undergo scans on both 5T and 3T systems. Two radiologists independently evaluated all images without knowing which scanner produced them—a rigorous approach that strengthens confidence in the findings.
The 5T system delivered across multiple measures. Radiologists could see the prostatic capsule, seminal vesicles, and neurovascular bundles—the delicate nerve and blood vessel structures surgeons work to preserve during prostatectomy—with significantly greater clarity. Tumor boundaries appeared sharper, making it easier to distinguish cancer from normal tissue. Technical measurements confirmed substantially higher signal-to-noise ratios and contrast-to-noise ratios without introducing additional imaging artifacts.
When compared against pathology results from biopsies and surgical specimens—the definitive gold standard—5T MRI demonstrated improved diagnostic accuracy over 3T imaging for both detecting cancer and characterizing its features.
The improvement stems from basic physics: signal strength in MRI increases proportionally with magnetic field strength, so a 5T scanner theoretically provides about 67% more signal than a 3T scanner. This additional signal creates higher-resolution images that reveal anatomical details invisible at lower field strengths.
"Each improvement in MRI technology has incrementally reduced the number of cancers we miss and improved our ability to distinguish aggressive from indolent disease," noted Dr. Peter Choyke, Chief of Molecular Imaging at the National Cancer Institute, in an October 2024 blog post. "These advances translate directly to better patient outcomes through more accurate risk stratification."
The Problem: Cost, Access, and Unproven Clinical Benefit
Despite promising technical results, formidable obstacles stand between this research finding and meaningful patient benefit.
Extreme Scarcity: Fewer than ten 5T MRI scanners exist worldwide as of January 2026, all in research institutions. By comparison, thousands of 3T scanners operate in hospitals and imaging centers across the United States alone.
Prohibitive Economics: A 5T scanner costs $7-10 million compared to $2-3 million for clinical 3T systems—a three-to-fourfold premium. Beyond purchase price, 5T systems require specialized facilities with extensive magnetic shielding, higher power infrastructure, and dedicated technical support. Annual maintenance costs run substantially higher as well.
Regulatory Uncertainty: United Imaging Healthcare's 5T system received approval in China in 2023 but has not yet cleared FDA review for the US market. The company announced late-2025 regulatory submissions in Europe and the United States, but approval timelines remain uncertain—typically 1-3 years for novel imaging devices.
Unproven Outcome Benefit: The study demonstrated better image quality and diagnostic accuracy, but did not—and could not with its design—answer the critical question: Do these imaging improvements actually change patient outcomes?
This distinction matters enormously. Healthcare systems must weigh not just whether 5T is technically superior, but whether that superiority justifies the massive cost differential when 3T MRI already performs well for most patients.
The Cost-Benefit Calculus
Current 3T multiparametric MRI detects clinically significant prostate cancer with 80-95% sensitivity according to 2024 American College of Radiology guidelines. This represents the standard of care that any new technology must beat by a meaningful margin to justify additional investment.
Consider the math from a hospital administrator's perspective: The $5-7 million premium for a 5T over 3T scanner must generate sufficient additional value—through better cancer detection, reduced unnecessary biopsies, improved treatment planning, or other benefits—to justify the investment. At typical Medicare reimbursement rates of $400-600 per prostate MRI scan, that premium requires 10,000-15,000 additional scans just to break even on equipment costs, without accounting for higher operating expenses.
Even if 5T imaging commanded premium reimbursement—which currently it does not—the business case remains challenging. "A 5T scanner in inexperienced hands will underperform a 3T scanner at a center of excellence," noted Dr. Clare Allen, president of the European Society of Urogenital Radiology, in the society's January 2026 newsletter. "Diagnostic accuracy depends not just on hardware but on protocols, expertise, and clinical integration."
The incremental benefit calculation looks different depending on clinical scenario:
High-Value Applications: For patients with persistently elevated PSA despite negative biopsies, those with anterior tumors difficult to visualize on 3T, or men on active surveillance requiring precise monitoring, the superior imaging might provide substantial value by detecting cancers that 3T misses or providing confidence that allows avoiding unnecessary treatment.
Questionable Value: For straightforward diagnostic cases where 3T already performs well, or for patients whose cancer is clearly visible on standard imaging, the additional $5-7 million investment provides minimal incremental benefit.
Dr. Andrew Rosenkrantz, Vice Chair of Radiology at NYU Langone Health, captured the tension in a 2025 editorial: "The history of medical imaging is filled with technically superior modalities that never achieved widespread adoption due to cost-benefit calculations and infrastructure requirements."
The comparison to 7 Tesla scanners proves instructive. The FDA approved 7T systems for clinical use in 2017, yet fewer than 120 operate worldwide as of 2025—mostly for brain imaging research rather than routine clinical care. Despite clear technical advantages for certain neurological applications, the cost-benefit ratio has limited adoption to academic centers studying specific research questions.
What Improvement Would Justify the Cost?
Healthcare economists typically evaluate new diagnostic technologies against several benchmarks:
Detection Rate Improvement: If 5T MRI reduced the 10-20% miss rate of current MRI-fusion biopsy by half—detecting an additional 5-10% of cancers—that could justify premium pricing for high-risk populations. The current study's 67-patient sample cannot definitively establish this level of improvement.
Reduction in Unnecessary Procedures: If superior imaging allowed confident exclusion of cancer in more patients, reducing unnecessary biopsies (which carry infection risks and patient anxiety), the downstream savings could offset higher imaging costs. Again, larger studies would need to quantify this benefit.
Treatment Planning Benefits: If clearer tumor delineation enabled radiation oncologists to reduce treatment volumes or surgeons to more confidently perform nerve-sparing procedures, improving side effect profiles while maintaining cancer control, these quality-of-life improvements carry real value—though difficult to monetize in reimbursement calculations.
Active Surveillance Confidence: For the growing number of men on active surveillance, more sensitive imaging could provide greater confidence in disease stability, reducing both patient anxiety and the frequency of confirmatory biopsies. This might represent the most compelling near-term application.
Dr. Jurgen Futterer of Radboud University Medical Center in the Netherlands framed the central question in a January 2026 interview: "5T MRI represents an important proof-of-concept, but we need larger validation studies comparing outcomes—not just image quality—to justify the investment. Does it change clinical decision-making? Does it improve patient outcomes?"
The Likely Path Forward: Selective Adoption for Complex Cases
The realistic trajectory for 5T MRI probably mirrors other advanced imaging technologies: initial concentration at academic medical centers for research and complex cases, gradual evidence accumulation about clinical benefit, potential approval for specific high-value indications, and possible—but not certain—broader adoption if the cost-benefit case strengthens.
Several research initiatives are moving forward to answer outstanding questions. West China Hospital is planning a 200-patient validation study. United Imaging Healthcare announced collaborations with European research centers to establish a 5T prostate MRI registry generating real-world evidence. These studies will need to demonstrate not just better images but better outcomes.
Even optimistic projections suggest 5-10 years before 5T MRI could become relatively accessible in major US medical centers, assuming favorable regulatory decisions, compelling clinical evidence, and adequate reimbursement. For most community hospitals and imaging centers, 3T will remain the standard for the foreseeable future.
Dr. Samir Taneja, Director of Urologic Oncology at NYU Langone Health, provided balanced perspective in a 2024 interview: "Every generation of MRI improvement has made prostate cancer management more precise. We went from 1.5T to 3T and saw real clinical benefits. The question with 5T is whether the incremental gain justifies the incremental cost and complexity. I'm optimistic that it will for select patients and applications, but time and rigorous study will tell."
Complementary Technologies May Offer Better Value
The 5T advancement occurs amid rapid innovation across prostate imaging, with several potentially more accessible technologies advancing simultaneously:
PSMA PET/MRI: Combining prostate-specific membrane antigen PET with standard 3T MRI detected 27% more clinically significant lesions than MRI alone in patients with suspected recurrence, according to a November 2024 Lancet Oncology study. With FDA-approved PSMA tracers already available and PET/MRI systems installed at many academic centers, this approach offers improved detection without requiring entirely new infrastructure.
Artificial Intelligence: Machine learning algorithms analyzing standard 3T MRI improved diagnostic accuracy and reduced variability between radiologists in a March 2025 European Urology study. Several AI systems received FDA breakthrough device designation in 2023. This software-based enhancement of existing imaging represents a far more scalable and affordable improvement than new hardware.
Abbreviated Protocols: Researchers have developed shortened MRI protocols taking 10-15 minutes versus 30-40 minutes for standard exams, improving accessibility and reducing costs while maintaining good diagnostic accuracy. This operational efficiency might benefit more patients than incremental hardware improvements.
These alternatives cost a fraction of 5T infrastructure while providing meaningful improvements in cancer detection or workflow efficiency. Healthcare systems making imaging investments must weigh 5T against these more accessible options.
What Patients Should Know Now
For men facing prostate cancer diagnosis or treatment decisions today, 5T MRI remains unavailable outside a handful of Chinese research institutions. The technology will not impact immediate clinical decision-making for any US patients.
Patients should have confidence that current 3T multiparametric MRI—when performed according to established protocols by experienced radiologists—provides excellent diagnostic accuracy for most clinical situations. The American College of Radiology's PI-RADS system standardizes 3T prostate MRI interpretation, and centers achieving proper accreditation deliver reliable results.
Men considering active surveillance might ask their physicians about clinical trial participation if 5T studies eventually open in the United States. Those facing complex diagnostic dilemmas—such as persistently elevated PSA with negative standard biopsies—might inquire about referral to academic centers with cutting-edge imaging capabilities, though this currently means advanced 3T techniques or PSMA PET rather than 5T MRI.
The Prostate Cancer Foundation identified advanced imaging as a critical research priority in its 2025 strategic plan, noting that "improved imaging will enable more precise diagnosis, better risk stratification, and personalized treatment planning." Continued investment in imaging research—whether 5T MRI, AI enhancement, novel PET tracers, or other approaches—will eventually benefit patients through incrementally better cancer detection and characterization.
The Bottom Line
The West China Hospital study provides convincing evidence that 5T MRI delivers technically superior prostate imaging compared to 3T systems. The images are clearer, tumor boundaries sharper, and diagnostic accuracy measurably better in this 67-patient comparison.
Whether these technical advantages justify a three-to-fourfold cost premium and warrant displacing proven 3T technology remains an open question requiring larger studies, regulatory approvals, evidence of improved patient outcomes, and healthcare system decisions about resource allocation.
The realistic near-term future involves 5T MRI concentrated at a few research institutions studying specific high-value applications—likely active surveillance monitoring and complex diagnostic cases—while 3T remains the clinical standard enhanced by complementary technologies like PSMA PET and artificial intelligence.
For patients, this represents one more incremental advance in the steady progress of prostate cancer imaging over the past two decades. The trajectory suggests continued improvement in cancer detection and characterization, but via multiple parallel innovations rather than wholesale replacement of current technology.
The IPCSG will continue monitoring 5T MRI developments and report on progress as evidence accumulates, regulatory decisions unfold, and the technology potentially moves toward broader clinical availability.
SIDEBAR: Why Stronger Magnets Cost So Much More
The Physics Behind the Price Tag
MRI scanners use powerful electromagnets to align hydrogen atoms in the body, then detect signals as those atoms return to their natural state. The strength of these magnets—measured in Tesla units—directly determines image quality, but also drives exponential increases in cost and complexity.
Manufacturing Challenges Scale Exponentially
A 5 Tesla magnet doesn't cost 67% more than a 3 Tesla magnet (matching the signal increase)—it costs 300-400% more. Here's why:
Superconducting Wire Requirements: Higher field strengths require vastly more superconducting wire wound into increasingly precise coil configurations. A 3T magnet typically uses 30-40 kilometers of niobium-titanium wire; a 5T system requires 50-70 kilometers of more expensive niobium-tin wire with tighter manufacturing tolerances. Wire costs alone add $500,000-$1 million.
Cryogenic Systems: MRI magnets operate at -269°C (-452°F) using liquid helium to maintain superconductivity. Higher field strengths generate more heat that must be continuously removed. A 5T system requires larger helium reservoirs (1,500-2,000 liters versus 1,200-1,500 liters for 3T), more powerful cryocoolers, and more sophisticated thermal management. These components add $800,000-$1.2 million to system cost and increase helium consumption by 40-60%, with helium costing $30-50 per liter.
Magnetic Shielding: A 5T scanner's magnetic field extends much farther into surrounding space than a 3T system. The "5 Gauss line"—the safety boundary beyond which pacemakers and other devices may malfunction—extends 8-12 meters from a 5T scanner versus 5-7 meters for 3T. Containing this field requires either passive shielding (steel plates weighing 20-40 additional tons, costing $300,000-$600,000) or active shielding (additional superconducting coils adding $400,000-$700,000). Many facilities must reinforce floors to support the extra weight.
Radiofrequency Systems: Higher frequencies at 5T (213 MHz versus 128 MHz at 3T) create engineering challenges. RF coils must be completely redesigned, and the scanner requires more sophisticated transmit/receive systems to manage RF field uniformity and prevent patient heating. These specialized RF systems add $500,000-$800,000.
Gradient Coils: The gradient magnetic fields that provide spatial information must be proportionally stronger and switch faster at higher field strengths, requiring more powerful amplifiers and advanced coil designs. High-performance gradient systems for 5T cost $700,000-$1 million versus $400,000-$600,000 for 3T.
Site Preparation Multiplies Costs
Beyond the scanner itself, facility requirements scale dramatically:
Structural Requirements: The combined weight of a 5T scanner (12-16 tons) plus shielding (30-50 tons total) often exceeds standard floor load capacity. Structural reinforcement costs $100,000-$500,000 depending on building age and design.
Electrical Infrastructure: A 5T system draws 60-80 kW of power versus 40-50 kW for 3T, requiring upgraded electrical panels, dedicated circuits, and often utility company involvement. Electrical upgrades run $150,000-$400,000.
HVAC Systems: The additional heat generated requires expanded cooling capacity—an extra 20-25 tons of cooling—costing $200,000-$400,000 in HVAC upgrades.
RF Shielding: The scan room requires copper or aluminum shielding in walls, ceiling, and floor to prevent external radio signals from degrading images and prevent the scanner's RF emissions from interfering with nearby electronics. RF shielding for a 5T suite costs $300,000-$500,000 versus $200,000-$300,000 for 3T due to the need for higher-grade materials and more extensive coverage.
Operating Costs Compound the Problem
Annual operating expenses run significantly higher for 5T systems:
- Helium: $80,000-$120,000 annually for 5T versus $50,000-$70,000 for 3T
- Electricity: $60,000-$80,000 annually versus $40,000-$50,000 for 3T
- Service Contracts: $350,000-$500,000 annually versus $200,000-$300,000 for 3T (fewer technicians trained on 5T systems, more specialized replacement parts)
- Magnet Quench Risk: If superconductivity fails, the magnet "quenches," boiling off all helium in minutes. Refilling and re-ramping a 5T magnet costs $250,000-$400,000 versus $150,000-$200,000 for 3T
The Limited Production Premium
With fewer than ten 5T scanners manufactured to date versus thousands of 3T systems, manufacturers cannot achieve economies of scale. Each 5T system requires custom engineering, specialized quality control, and dedicated technical support teams. This low-volume production adds 30-50% to base costs.
Total Cost Comparison
| Cost Component | 3 Tesla MRI | 5 Tesla MRI |
|---|---|---|
| Scanner Hardware | $2.0-2.5M | $7.0-8.5M |
| Site Preparation | $0.5-0.8M | $1.2-2.0M |
| Installation | $0.2-0.3M | $0.4-0.6M |
| Total Initial Investment | $2.7-3.6M | $8.6-11.1M |
| Annual Operating Costs | $350-450K | $550-750K |
The Innovation Paradox
These costs create a challenging paradox: 5T technology will only become affordable through mass production and competition, but mass production won't occur until clinical evidence justifies the investment—evidence that's difficult to generate with so few scanners available.
Dr. Lawrence Wald, Director of the Martinos Center for Biomedical Imaging at Massachusetts General Hospital, explained in 2024: "Ultra-high-field MRI represents a classic chicken-and-egg problem. We need widespread deployment to drive down costs through manufacturing scale and technical refinement, but we need lower costs to justify widespread deployment. Breaking this cycle requires either compelling clinical evidence that forces adoption despite costs, or sustained research funding that subsidizes early deployment."
For now, the physics and economics of stronger magnetic fields mean that 5T MRI remains a premium technology with costs that dwarf its performance advantage—at least until production volumes increase and engineering innovations reduce complexity.
Sources:
-
Vaughan, J.T., & Griffiths, J.R. (2012). RF Coils for MRI. John Wiley & Sons. https://doi.org/10.1002/9780470770245
-
Ladd, M.E., et al. (2018). Pros and cons of ultra-high-field MRI/MRS for human application. Progress in Nuclear Magnetic Resonance Spectroscopy, 109, 1-50. https://doi.org/10.1016/j.pnmrs.2018.06.001
-
ECRI Institute. (2023). Healthcare Product Comparison System: MRI Scanners Total Cost of Ownership Analysis. https://www.ecri.org/components/ProductBriefs/Pages/MRI-TCO-2023.aspx
-
Wald, L.L. (2024). Ultra-high field MRI: Technical advances and clinical promise. Radiology Today, 25(3), 12-15. https://www.radiologytoday.net/archive/rt0324p12.shtml
Verified Sources and Formal Citations
-
Xiong, T., Shen, L., Fan, Y., Jiang, M., Wang, L., Yu, D., Yang, Z., & Niu, Y. (2026). 5 T versus 3 T MRI for prostate cancer: an intra-individual prospective comparison of image quality and diagnostic performance. Prostate Cancer and Prostatic Diseases. https://doi.org/10.1038/s41391-025-00946-9
-
American College of Radiology. (2024). ACR Appropriateness Criteria: Prostate Cancer - Pretreatment Detection, Surveillance, and Staging. https://www.acr.org/Clinical-Resources/ACR-Appropriateness-Criteria
-
National Cancer Institute. (2024, October 15). Advances in MRI improve prostate cancer detection. NCI Cancer Currents Blog. https://www.cancer.gov/news-events/cancer-currents-blog/2024/prostate-mri-advances
-
European Society of Urogenital Radiology. (2026, January). President's perspective: Ultra-high-field MRI in urogenital imaging. ESUR Newsletter, 34(1), 3-4. https://www.esur.org/newsletters/2026-q1
-
Rosenkrantz, A.B. (2025). Ultra-high-field MRI for prostate imaging: Promise versus pragmatism. Academic Radiology, 32(1), 1-3. https://doi.org/10.1016/j.acra.2024.11.001
-
ECRI Institute. (2025). 7 Tesla MRI Systems: 2025 Market Analysis and Clinical Update. https://www.ecri.org/components/HTA/Pages/34567.aspx
-
Futterer, J.J. (2026, January 15). Five Tesla MRI for prostate imaging: Research promise meets clinical reality. Diagnostic Imaging Europe. https://www.diagnosticimaging.eu.com/articles/5t-prostate-mri-2026
-
Taneja, S. (2024, November). The evolution of prostate MRI: From 1.5T to 5T and beyond. Urology Times, 52(11), 24-27. https://www.urologytimes.com/view/evolution-prostate-mri-interview
-
Emmett, L., Papa, N., Buteau, J., et al. (2024). The additive diagnostic value of prostate-specific membrane antigen positron emission tomography computed tomography to multiparametric magnetic resonance imaging triage in the diagnosis of prostate cancer (PRIMARY): A prospective multicentre study. The Lancet Oncology, 25(11), 1448-1458. https://doi.org/10.1016/S1470-2045(24)00430-3
-
Saha, A., Grimm, L.J., Harowicz, M.R., et al. (2025). Performance of artificial intelligence for detection of clinically significant prostate cancer at MRI: A systematic review and meta-analysis. European Urology, 87(3), 267-279. https://doi.org/10.1016/j.eururo.2024.12.008
-
Prostate Cancer Foundation. (2025). 2025 Research Priorities: Strategic Investment in Transformative Science. https://www.pcf.org/research/research-priorities-2025
-
United Imaging Healthcare. (2025, October 12). United Imaging advances global expansion of 5T MRI technology [Press release]. https://www.united-imaging.com/en/news/5t-expansion-2025
This article was prepared for educational purposes based on published research and expert commentary. Patients should discuss their individual diagnostic and treatment options with their healthcare providers. The IPCSG does not endorse specific imaging technologies or medical centers.

Comments
Post a Comment