Enzalutamide in metastatic hormone-sensitive prostate cancer: results from the international, multicentre, real-world ARON-3 study
Enzalutamide in metastatic hormone-sensitive prostate cancer: results from the international, multicentre, real-world ARON-3 study | Prostate Cancer and Prostatic Diseases
Real-World Evidence Confirms Enzalutamide's Strong Performance in Newly Diagnosed Metastatic Prostate Cancer
IPCSG Newsletter Article
January 2026
BLUF (Bottom Line Up Front)
The largest international real-world study of enzalutamide (Xtandi) in metastatic hormone-sensitive prostate cancer confirms the drug's excellent survival outcomes and favorable safety profile outside of clinical trials. The ARON-3 study of 424 patients across 9 countries found that achieving ultra-low PSA levels (≤0.2 ng/mL) within months of starting treatment strongly predicts long-term survival. Even elderly patients over 70 and those with poorer performance status—groups typically excluded from clinical trials—experienced good outcomes with manageable side effects. These findings validate enzalutamide as a strong first-line treatment option for men newly diagnosed with metastatic prostate cancer.
Introduction: Bridging the Gap Between Trials and Real Life
When your oncologist recommends a cancer treatment, that recommendation is typically based on results from carefully controlled clinical trials. But clinical trials have strict entry criteria that often exclude older patients, those with other health conditions, or people who don't fit the "ideal" patient profile. This raises an important question: Do these drugs work as well in the real world as they do in trials?
The ARON-3 study helps answer this question for enzalutamide (marketed as Xtandi), one of the newer hormone therapies used to treat metastatic hormone-sensitive prostate cancer (mHSPC)—the stage when cancer has spread beyond the prostate but still responds to testosterone suppression. Published in January 2026 in the journal Prostate Cancer and Prostatic Diseases, this international study tracked 424 men across 29 cancer centers in 9 countries who received enzalutamide as part of their initial treatment for metastatic disease.
What the Study Found: Strong Survival and Good Tolerability
Survival Outcomes Mirror Clinical Trials
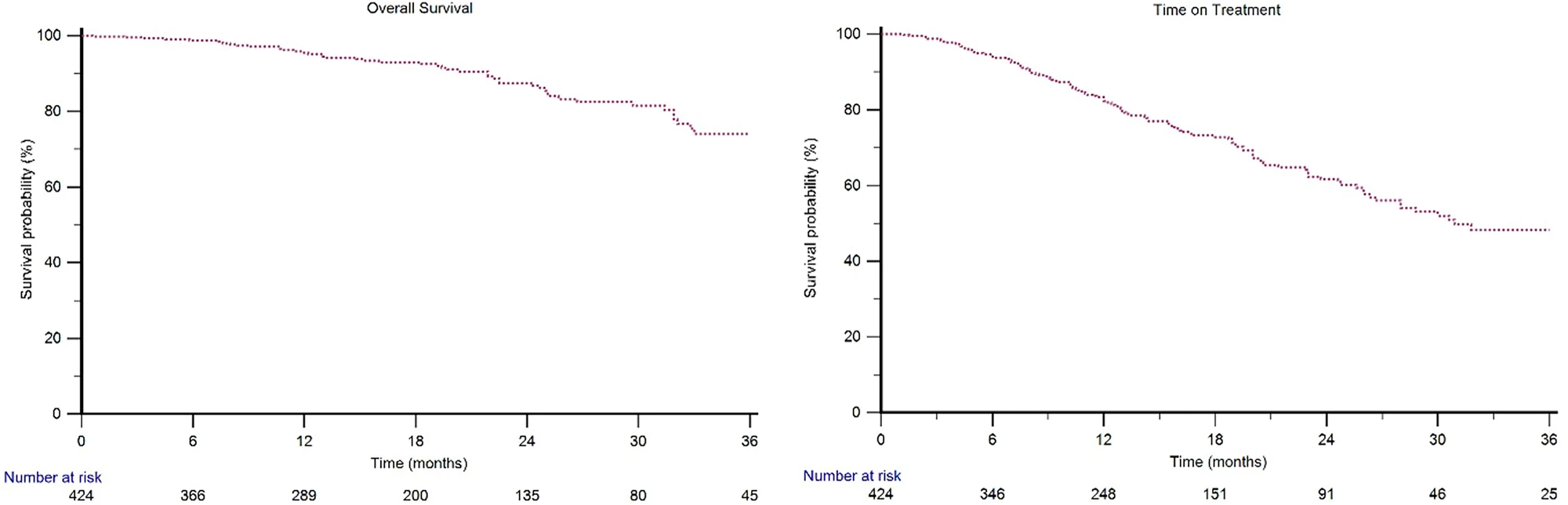
The ARON-3 study's results closely matched those of the ARCHES clinical trial, which originally demonstrated enzalutamide's benefits in this setting. After a median follow-up of 18.7 months:
- Median overall survival was not yet reached, meaning more than half of patients were still alive
- Two-year survival rate was 87% overall
- Only 13% of patients (53 out of 424) had died at the time of analysis
These numbers are remarkably similar to the ARCHES trial, which showed an 86% two-year survival rate. This is important because it demonstrates that enzalutamide works just as well in everyday clinical practice as it does in the controlled environment of a clinical trial.
Treatment Duration Provides Real-World Insight
One of the most practical measures in real-world studies is "time on treatment" (ToT)—how long patients actually stay on the medication before stopping for any reason, including side effects or disease progression. The median ToT was 31.8 months (nearly three years), indicating that most men could tolerate the treatment well for an extended period.
Treatment duration was particularly impressive for certain patient groups:
- Patients with lymph node-only metastases: Median ToT not yet reached
- Patients with low-volume disease: 43.3 months median ToT
- Patients with good performance status (ECOG-PS = 0): Median ToT not yet reached
PSA Response: A Powerful Predictor
One of the study's most clinically useful findings involves PSA (prostate-specific antigen) response patterns. The researchers looked at two key PSA milestones:
PSA90: A decrease of 90% or more from baseline PSA
PSA0.2: Achieving an ultra-low PSA of 0.2 ng/mL or less
The results were striking:
- 76% of patients achieved PSA90 (median time: 6 months)
- 59% achieved PSA0.2 (median time: 8.3 months)
- Patients who reached PSA0.2 had a 96% two-year survival rate compared to 78% for those who didn't
This finding gives oncologists and patients an early indicator of treatment effectiveness. If your PSA drops to very low levels within the first several months of treatment, it's a strong sign that you're likely to do well long-term.
Safety Profile Remains Favorable
Grade 3-4 adverse events (the most severe side effects) occurred in only 9% of patients. The most common severe side effect was fatigue (9%), with hypertension reported in 2%. Importantly, only 9% of patients needed to reduce their dose due to side effects.
Even among patients over 70 years old—a group often more vulnerable to side effects—the safety profile remained good, with a 10% rate of severe adverse events. This is particularly reassuring since 45% of the study population had some degree of functional impairment (ECOG-PS > 0), and 7% had significant limitations (ECOG-PS = 2)—patients who would have been excluded from the original ARCHES trial.
Who Benefits Most? Patient Subgroup Insights
Disease Volume and Location Matter
The study confirmed that the extent and location of metastatic disease significantly affects outcomes:
Best outcomes (in order):
- Lymph node-only metastases (M1a): 98% two-year survival
- Bone metastases (M1b): 88% two-year survival
- Visceral metastases (M1c): 80% two-year survival
Patients with low-volume disease according to CHAARTED criteria (fewer than four bone metastases and no visceral involvement beyond lymph nodes) had longer treatment duration than those with high-volume disease (43.3 months vs. 28.0 months).
Visceral Metastases Present Challenges
The presence of metastases in organs like the lungs or liver was associated with poorer outcomes:
- Lung metastases: Nearly 3 times higher risk of death
- Liver metastases: More than 13 times higher risk of death
These findings suggest that men with visceral metastases, particularly liver involvement, might benefit from more intensive treatment approaches, such as the "triple therapy" regimens that combine hormone therapy, enzalutamide (or similar drugs), and chemotherapy.
Age Is Just a Number—Mostly
Younger patients (under 70) did have better overall survival compared to older patients, but the difference wasn't dramatic. More importantly, the treatment was well-tolerated across all age groups, with elderly patients experiencing only marginally more side effects.
Prior Chemotherapy Affects Duration
The 17% of patients who received docetaxel chemotherapy before starting enzalutamide had a shorter median time on treatment (23.0 months vs. 36.1 months). However, their two-year survival rate of 76% was still respectable, indicating that enzalutamide remains a valuable option even after chemotherapy.
What This Means for Treatment Decisions
Real-World Validation Builds Confidence
The ARON-3 study's close alignment with the ARCHES trial results provides strong reassurance that enzalutamide's benefits translate to everyday clinical practice. This is especially meaningful for patients who might not have qualified for the original clinical trials due to age, performance status, or other factors.
PSA Response as a Monitoring Tool
The strong association between achieving ultra-low PSA levels and improved survival offers a practical monitoring strategy. While PSA isn't perfect, these results suggest that if your PSA drops to very low levels (≤0.2 ng/mL) within the first several months of treatment, you're likely responding well to therapy.
It's important to note, however, that PSA response takes time with enzalutamide. The median time to PSA0.2 was 8.3 months, so patience is warranted. A slower PSA decline doesn't necessarily mean treatment failure.
Treatment Selection Considerations
While the study focused on enzalutamide, several similar drugs are available for newly diagnosed metastatic prostate cancer, including apalutamide (Erleada), darolutamide (Nubeqa), and abiraterone acetate (Zytiga). Direct head-to-head comparisons between these agents are limited, making treatment selection often a matter of:
- Side effect profiles
- Drug interactions
- Cost and insurance coverage
- Patient and physician preference
Enzalutamide's demonstrated low rate of severe side effects (particularly skin reactions and cardiovascular events) may make it preferable for patients who are particularly concerned about tolerability.
The Case for Triple Therapy
The study's findings on disease volume reinforce current treatment trends. For men with high-volume metastatic disease, particularly those with bone and visceral involvement, combining hormone therapy, an androgen receptor pathway inhibitor (like enzalutamide), and docetaxel chemotherapy upfront—so-called "triple therapy"—has shown substantial benefits in recent trials.
The ARON-3 data suggests that men with low-volume disease or lymph node-only metastases may do very well with the doublet approach (hormone therapy plus enzalutamide), potentially sparing them the additional toxicity of immediate chemotherapy.
Understanding the Study's Limitations
While the ARON-3 study provides valuable real-world data, it has some important limitations:
Follow-up Duration: The median follow-up of 18.7 months is relatively short compared to the ARCHES trial's 44.6 months. Longer-term outcomes will become clearer with additional follow-up.
Retrospective Design: This was not a randomized controlled trial. Doctors and patients chose enzalutamide for various reasons, which could introduce selection bias.
Missing Data: As with most real-world studies, some information was incomplete. For example, the study didn't consistently capture:
- Type of imaging used to detect metastases (conventional vs. PSMA PET scans)
- Patient comorbidities (other health conditions)
- Some quality-of-life measures
No Direct Comparisons: The study didn't compare enzalutamide to other treatment options, so we can't determine from this data whether one drug is superior to another.
Potential Underreporting: Adverse events may be underreported in real-world studies compared to clinical trials, where monitoring is more systematic.
The Bigger Picture: Where Treatment Is Heading
The Evolution of Metastatic Prostate Cancer Treatment
Just a decade ago, the standard treatment for newly diagnosed metastatic prostate cancer was androgen deprivation therapy (ADT) alone—chemical or surgical castration to lower testosterone levels. Patients typically stayed on ADT until it stopped working, then moved to chemotherapy or other treatments.
The landscape has transformed dramatically. Multiple studies have now shown that adding drugs like enzalutamide to ADT from the very beginning extends survival significantly. The ARCHES trial demonstrated a 34% reduction in the risk of death with enzalutamide plus ADT compared to ADT alone.
Triple Therapy and Treatment Intensification
More recently, studies like PEACE-1 (with abiraterone) and ARASENS (with darolutamide) have shown that adding chemotherapy to the combination—creating "triple therapy"—provides even greater benefits, particularly for men with high-volume disease.
This raises important questions about how to sequence therapies and which patients need the most intensive upfront treatment. The ARON-3 findings on disease volume and metastatic patterns help inform these decisions.
Biomarkers and Personalized Treatment
The strong prognostic value of PSA response in this study aligns with growing interest in using biomarkers to guide treatment. While not yet standard practice, there's increasing research into:
- Circulating tumor DNA
- AR-V7 testing
- Genomic profiling of tumors
- Advanced imaging techniques
These tools may eventually help identify which patients need treatment intensification and which can be managed with less aggressive approaches.
Questions to Discuss with Your Oncologist
If you've been diagnosed with metastatic hormone-sensitive prostate cancer, consider discussing these questions with your medical team:
-
Based on my disease volume and metastatic sites, would you recommend doublet therapy (ADT + ARPI) or triple therapy (ADT + ARPI + chemotherapy)?
-
What are the key differences in side effect profiles between the available ARPIs (enzalutamide, apalutamide, darolutamide, abiraterone)?
-
How frequently should we monitor my PSA, and what PSA targets should we aim for?
-
If my PSA doesn't reach ultra-low levels, what would be the next steps?
-
How does my age, performance status, and other health conditions factor into the treatment recommendation?
-
What are the pros and cons of adding chemotherapy to my initial treatment regimen?
-
Are there any clinical trials I should consider?
Conclusion: Evidence-Based Optimism
The ARON-3 study reinforces that we're in an era of substantially improved outcomes for metastatic prostate cancer. Enzalutamide, combined with standard androgen deprivation therapy, provides strong survival benefits with a generally manageable side effect profile for the vast majority of patients—including older men and those with some functional limitations.
The identification of ultra-low PSA achievement as a strong predictor of outcomes gives patients and physicians a concrete early indicator of treatment success. While not everyone will achieve these ultra-low levels, those who do can take encouragement from the excellent long-term outcomes associated with this response.
As always, treatment decisions should be individualized based on disease characteristics, overall health status, personal preferences, and practical considerations like cost and quality of life. The expanding treatment options for metastatic prostate cancer mean that there are now multiple effective pathways, and ongoing research continues to refine our understanding of how to best deploy these therapies.
For men living with metastatic prostate cancer, the message from ARON-3 is one of evidence-based optimism: modern treatments work, they work in real-world settings, and they're extending lives while maintaining quality of life.
Verified Sources and Formal Citations
-
Büttner T, Rizzo M, Bernal Vaca L, et al. "Enzalutamide in metastatic hormone-sensitive prostate cancer: results from the international, multicentre, real-world ARON-3 study." Prostate Cancer and Prostatic Diseases (2026). https://doi.org/10.1038/s41391-025-01067-3
[Published January 8, 2026] -
Armstrong AJ, Azad AA, Iguchi T, et al. "Improved survival with enzalutamide in patients with metastatic hormone-sensitive prostate cancer." Journal of Clinical Oncology 40, no. 15 (2022): 1616-1622. https://doi.org/10.1200/JCO.22.00193
[ARCHES trial final overall survival analysis] -
Armstrong AJ, Szmulewitz RZ, Petrylak DP, et al. "ARCHES: a randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer." Journal of Clinical Oncology 37, no. 32 (2019): 2974-2986. https://doi.org/10.1200/JCO.19.00799
[Original ARCHES trial publication] -
Stenzl A, Shore ND, Villers A, et al. "Clinical outcomes of patients with metastatic Hormone-Sensitive Prostate Cancer (mHSPC) with Prostate-Specific Antigen (PSA) decline to undetectable levels on enzalutamide (ENZA): Post hoc analysis of ARCHES." European Urology 81, Suppl. (2022): S776-S777.
[ARCHES PSA response analysis] -
Fizazi K, Foulon S, Carles J, et al. "Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): a multicentre, open-label, randomised, phase 3 study with a 2 × 2 factorial design." Lancet 399, no. 10336 (2022): 1695-1707. https://doi.org/10.1016/S0140-6736(22)00367-1
[Triple therapy trial with abiraterone] -
Smith MR, Hussain M, Saad F, et al. "Darolutamide and survival in metastatic, hormone-sensitive prostate cancer." New England Journal of Medicine 386, no. 12 (2022): 1132-1142. https://doi.org/10.1056/NEJMoa2119115
[ARASENS trial with darolutamide triple therapy] -
Chowdhury S, Bjartell A, Agarwal N, et al. "Deep, rapid, and durable prostate-specific antigen decline with apalutamide plus androgen deprivation therapy is associated with longer survival and improved clinical outcomes in TITAN patients with metastatic castration-sensitive prostate cancer." Annals of Oncology 34, no. 5 (2023): 477-485. https://doi.org/10.1016/j.annonc.2023.02.009
[TITAN trial PSA response analysis with apalutamide] -
Santoni M, Büttner T, Rescigno P, et al. "Apalutamide in metastatic castration-sensitive prostate cancer: results from the multicenter real-world ARON-3 study." European Urology Oncology 8, no. 2 (2025): 444-451.
[Companion ARON-3 analysis on apalutamide] -
Sweeney CJ, Chen YH, Carducci M, et al. "Chemohormonal therapy in metastatic hormone-sensitive prostate cancer." New England Journal of Medicine 373, no. 8 (2015): 737-746. https://doi.org/10.1056/NEJMoa1503747
[CHAARTED trial establishing disease volume criteria] -
Matsubara N, Chi KN, Özgüroğlu M, et al. "Correlation of prostate-specific antigen kinetics with overall survival and radiological progression-free survival in metastatic castration-sensitive prostate cancer treated with abiraterone acetate plus prednisone or placebos added to androgen deprivation therapy: post hoc analysis of phase 3 LATITUDE study." European Urology 77, no. 4 (2020): 494-500. https://doi.org/10.1016/j.eururo.2019.11.021
[PSA kinetics in LATITUDE trial] -
Tran C, Ouk S, Clegg NJ, et al. "Development of a second-generation antiandrogen for treatment of advanced prostate cancer." Science 324, no. 5928 (2009): 787-790.
[Enzalutamide mechanism of action - foundational research] -
Azad AA, Armstrong AJ, Alcaraz A, et al. "Efficacy of enzalutamide in subgroups of men with metastatic hormone-sensitive prostate cancer based on prior therapy, disease volume, and risk." Prostate Cancer and Prostatic Diseases 25, no. 2 (2022): 274-282. https://doi.org/10.1038/s41391-021-00436-y
[ARCHES subgroup analyses] -
Assayag J, Kim C, Chu H, Webster J. "The prognostic value of Eastern Cooperative Oncology Group performance status on overall survival among patients with metastatic prostate cancer: a systematic review and meta-analysis." Frontiers in Oncology 13 (2023): 1194718. https://doi.org/10.3389/fonc.2023.1194718
[Performance status as prognostic factor]
About the ARON-3 Study: The ARON-3 database represents a collaborative international effort involving 29 oncology centers across 9 countries (Italy, Spain, Colombia, Czech Republic, Belgium, Poland, Argentina, Mexico, Germany, United Kingdom, United States, and Turkey). The study was approved by the Ethics Committee of the Marche Region, Italy (No. 2024 20) and was registered at ClinicalTrials.gov (identifier: NCT06200558). The protocol followed the Declaration of Helsinki and Good Clinical Practice standards.
Funding Disclosure: Partial support was provided by NCI grant P30 CA077598 and DOD grant W81XWH-22-2-0025 (not directly related to this specific analysis).
This article is intended for educational purposes and should not replace consultation with your healthcare provider. Treatment decisions should be made in partnership with your oncology team based on your individual circumstances.
Prepared for the Informed Prostate Cancer Support Group (IPCSG)
January 2026

Comments
Post a Comment